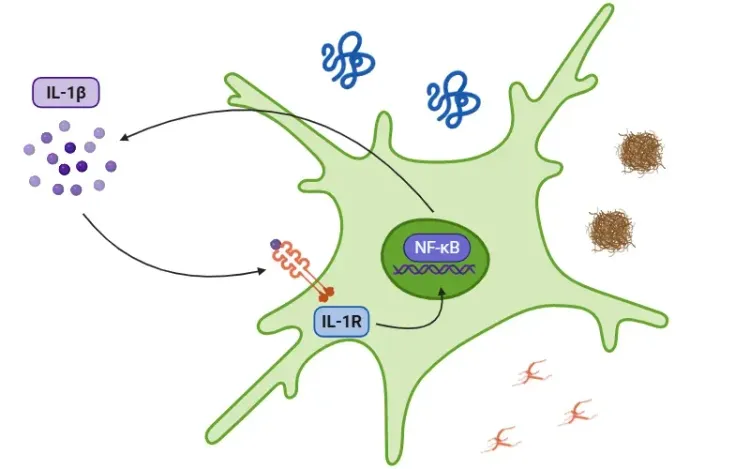
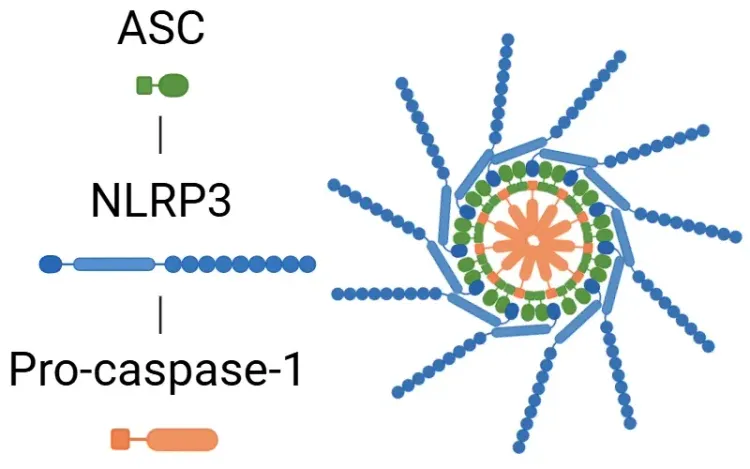
What is IL-1β?
IL-1β (IL-1b; Interleukin-1 Beta) is a pro-inflammatory cytokine encoded by the IL1B gene and produced mainly by activated macrophages. It is synthesized as an inactive precursor and becomes biologically active after cleavage by inflammasome-associated caspases, enabling it to drive inflammatory immune responses.
Interleukin-1 Beta is a single-chain protein considered a pro-inflammatory cytokine. It belongs to the IL-1 superfamily, which comprises various cytokines with a molecular weight of approximately 17-18 kDa, including IL-1α, IL-1Ra, IL-18, IL-33, and IL-38. The primary sources of IL-1β production are monocytes, macrophages, and neutrophils. In the brain, it is secreted by microglia and astrocytes. While IL-1β is widely expressed throughout the body, it is found in particularly high concentrations in:
- Bone marrow
- Urinary tract
- Brain
- Gastrointestinal tract
- Connective and soft tissues
- Liver
- Gallbladder
(Kaneko, 2019; Boraschi, 2022)
IL-1β plays various roles in the body, contributing to homeostatic immune responses and playing a part in essential physiological processes like sleep, pain, and neuroplasticity (Ren & Torres, 2009). Its primary function lies in promoting inflammation during pathological conditions. IL-1β is involved in both innate and adaptive immunity and can stimulate fever by increasing the levels of COX-2 and PGE2 in the hypothalamus (Boraschi, 2022).
The signaling cascade is initiated as a response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), detected by toll-like receptors (TLRs). IL-1β secretion occurs through pathways that are either dependent or independent of the inflammasome.
In the inflammasome pathway, the activation of the NF-κB transcription factor by PAMPs or DAMPs leads to the release of pro-IL-1β, pro-IL-18, and NLRP3. NLRP3 then recruits ASC and pro-caspase-1 to form the inflammasome, which activates caspase-1. This enzyme cleaves IL-1β and IL-18 into their active forms, thus promoting inflammation (Lopez-Castejon & Brough, 2011; Martin-Sanchez, 2016; Swanson, 2019).
For more details on the NLRP3 inflammasome, see: "What is NLRP3?"
On the other hand, pro-IL-1β can be cleaved into IL-1β by myeloblastin in neutrophils. Regardless of the pathway, the result of IL-1β secretion triggers a cascade of kinases, including TAK1 and MAPK, which activate pro-inflammatory transcription factors, such as NF-κB and AP-1. This process ultimately leads to the increased release of pro-inflammatory signals, including additional IL-1β, IL-18, and NLRP3 (Weber, 2010; Kaneko, 2019; Ford, 2025).
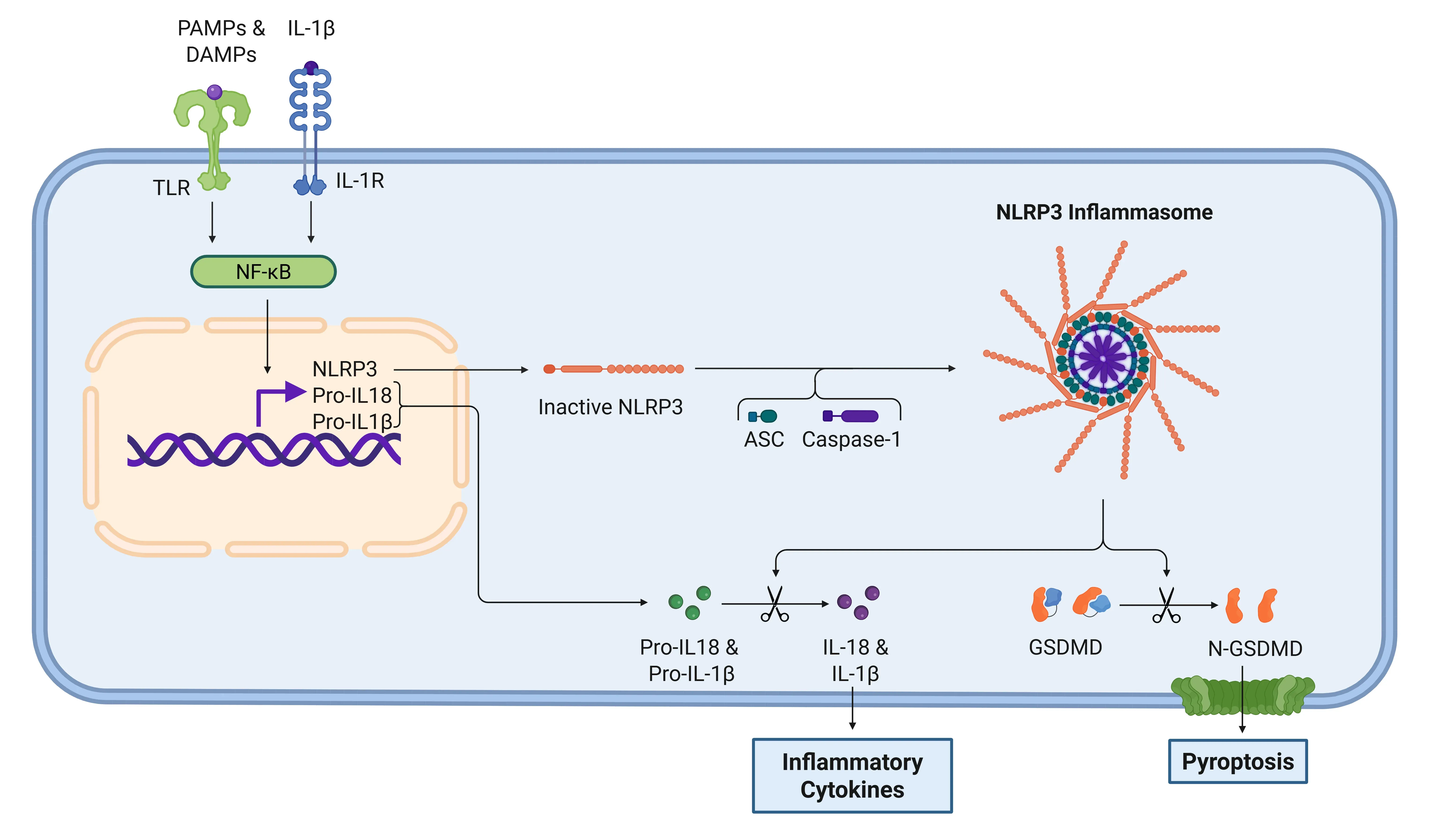
IL-1β signaling cascade via NLRP3 inflammasome.
Detection of PAMPs and DAMPs by TLRs activates NF-κB, inducing expression of NLRP3 and pro-forms of IL-1β and IL-18. Subsequent inflammasome assembly activates caspase-1, which cleaves pro-cytokines into their active forms and processes gasdermin D (GSDMD), leading to pyroptosis. IL-1β then signals through IL-1R to amplify the inflammatory response.
Because of IL-1β secretion, there is an overall increase in the inflammatory response. Several target genes are transcribed, including:
- IL-1α
- IL-17
- COX2
- PGE2
- RANKL
- MKP1
- IL-1β
Furthermore, the inflammatory cascade leads to increased capillary permeability, allowing immune cells to extravasate into affected tissues, further promoting inflammation. The production of IL-1β and IL-18 also serves to alert other immune cells, while simultaneously modulating the activities of neutrophils, mast cells, Th17 cells, and osteoclasts (Sakurai, 2012; Ford, 2025).
What is the role of IL-1β in systemic and neurodegenerative diseases?
While IL-1β plays a crucial role in promoting acute inflammation, elevated levels can lead to excessive inflammatory responses, contributing to various diseases. Conditions such as gout and juvenile idiopathic arthritis are mediated by IL-1β, and increased levels have also been observed in osteoarthritis (Dinarello, 2019). Furthermore, IL-1β can stimulate the development of immunosuppressive myeloid-derived suppressor cells (MDSCs) and tumour-associated macrophages (TAMs), which not only promote tumor inflammation, but also aid in immune evasion (Bent, 2018; Kaneko, 2019). These TAMs have been identified across multiple human cancers, including pancreatic and colon cancers, and are associated with poorer patient prognosis (Caronni, 2025).
IL-1β is also implicated in neurodegenerative diseases by perpetuating the immune response. It is involved in the NLRP3 inflammasome pathway, significantly contributing to neuroinflammation (Mendiola & Cardona, 2018).
For more on the role of the NLRP3 Inflammasome in neurodegenerative diseases, see: NLRP3 Inflammasome and Neurodegenerative Diseases
Interestingly, some studies suggest that IL-1β may also exert neuroprotective effects through its interaction with the IL-1R1+IL-1R3b receptor, and it is involved in memory and learning through its actions on the IL-1R9 receptor (Boraschi, 2023).
For a comprehensive review of the role of IL-1β in neurodegenerative diseases, see: Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
Alzheimer’s disease (AD)
Cytokines of the IL-1 superfamily are highly involved in inflammation in AD, particularly IL-1β and IL-18. Elevated levels of IL-1β can be found in cerebrospinal fluid (CSF), likely due to the activation of the NLRP3 inflammasome, which is triggered by amyloid-beta (Aβ) (Mendiola, 2018; Xu, 2025). The release of IL-1β sets off a detrimental cycle that not only enhances the production of Aβ but also promotes the formation of neurofibrillary tangles and further inflammasome activation. Conversely, IL-1Ra, another cytokine in the IL-1 superfamily, acts as a crucial protective factor. Research indicates that the absence of IL-1Ra in mouse models worsens the disease pathology (Boraschi, 2023).
Parkinson’s disease (PD)
PD is characterized by the misfolding of α-synuclein and the degeneration of dopaminergic neurons, which trigger the activation of the NLRP3 inflammasome (Dzamko, 2023; Xu, 2025). The NLRP3 inflammasome plays a significant role in the development of PD by producing IL-1β, a key inflammatory mediator (Boraschi, 2023). In patients with PD, levels of cytokines, such as IL-1β, TNFα, and IL-6, are elevated in CSF, contributing to the neuroinflammatory processes associated with the disease.
For more on the role of TNFα in neurodegenerative diseases, see:
Overexpression of IL-1β in the substantia nigra leads to loss of dopamine neurons and increased neuroinflammation, whereas overexpression in the hippocampus results in cognitive impairment (Dzamko, 2023). Nevertheless, the precise role of IL-1β in the context of PD is still not fully understood (Boraschi, 2023).
See: Microglia, Astrocytes & α-Synuclein in Parkinson’s disease & AAV α-Synuclein Models for Parkinson’s Disease Drug Development
Multiple Sclerosis (MS)
Patients with MS show elevated levels of caspase-1, IL-1β, and IL-18. The NLRP3 inflammasome plays a significant role in the development of MS by promoting the migration of CD4(+) T cells. In cases of primary progressive MS, there is an overproduction of the NLRP3 inflammasome by monocytes, which in turn boosts the production of IL-1β (Mendiola & Cardona, 2018; Xu, 2025). In the context of Experimental Autoimmune Encephalomyelitis (EAE), IL-1β is secreted from myeloid cells that have been recruited by IL-17A through a pathway that does not involve the inflammasome (McGinley, 2020). This IL-1β not only increases the permeability of the blood-brain barrier but also facilitates the recruitment of leukocytes, primarily by affecting astrocytes and endothelial cells (Boraschi, 2023).
For a summary of the animal models used to study MS, see: Multiple Sclerosis Models
Amyotrophic Lateral Sclerosis (ALS)
ALS is characterized by the presence of TDP-43, which is a key pathological marker in both ALS and frontotemporal dementia. TDP-43 inclusions are known to activate the NLRP3 inflammasome in primary microglial cultures, resulting in an increased production of IL-1β (Bright, 2021).
For additional information on ALS and the role of TDP-43, refer to ALS Mouse Models & Spinal Motor Neurons & ALS Mouse Models for Drug Development
IL-1β is also involved in the inflammation associated with mutant superoxide dismutase (SOD1), one of the two most common mutations associated with ALS. SOD1 can also activate the inflammasome, triggering neuroinflammation. Interestingly, IL-1β can act as a biomarker for ALS and has been found to have a negative correlation with patient survival, particularly in those with the specific genetic variant, C9orf72HRE (Dutta, 2020; Boraschi, 2023).
Could IL-1β serve as a therapeutic target?
IL-1β is recognized as a promising therapeutic target in various diseases, primarily due to its significant role in inflammation. IL-1 receptor antagonists (IL-1Ra) have shown promising results in reducing disease severity and improving outcomes in autoimmune conditions and show potential for neurodegenerative disorders.
Some notable therapies targeting IL-1β include:
- Anakinra:
- The intrinsic human IL-1Ra. This treatment inhibits the activity of both IL-1α and IL-1β and has received approval for use in autoinflammatory diseases such as rheumatoid arthritis, neonatal-onset multisystem inflammatory disease (NOMID), and deficiency of interleukin-1 receptor antagonist (DIRA) (Ford, 2025). Anakinra is the most extensively studied IL-1Ra medication and may offer benefits in neurodegenerative diseases, although research in this area remains limited.
- In animal models of AD, Anakinra has been shown to improve cognitive impairment by reducing IL-1β levels and decreasing amyloid-beta and tau deposits (Xu, 2025). Conversely, while blocking IL-1Ra in mouse models of ALS has been shown to prolong survival, it has not impacted disease onset. Although safety and tolerability studies for treating ALS have been conducted in humans, further clinical trials are needed to assess the efficacy of this treatment (Maier, 2015).
- Rilonacept:
- Consists of the extracellular portion of human IL-1R1 and IL-1R3 fused with the Fc portion of human IgG1. This design allows for long-term inhibition of both IL-1α and IL-1β, and Rilonacept has been approved for the treatment of cryopyrin-associated periodic syndromes (CAPS).
- Canakinumab: A human monoclonal IgG1 antibody that specifically targets IL-1β, without affecting IL-1α or IL-1R1. It has received approval for treating Still’s disease, periodic fever syndromes, and gout flares (Dinarello, 2019).
Together, these therapies highlight the potential of targeting IL-1β in managing inflammatory conditions, offering new alternatives for treating a range of neurodegenerative diseases.
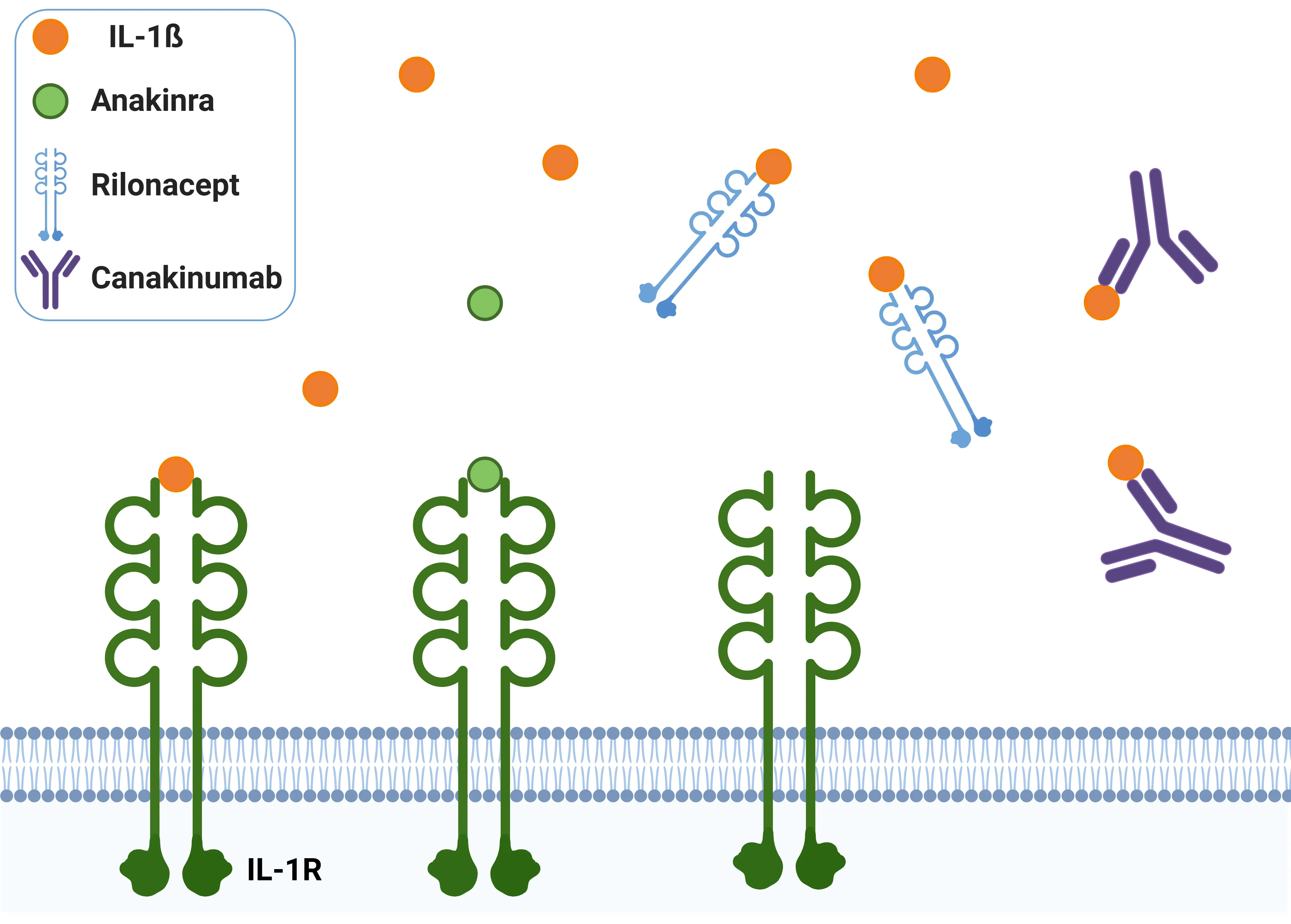
IL-1β antagonists.
Anakinra blocks IL-1β by competitively binding IL-1R. Rilonacept acts as a decoy receptor for IL-1β. Canakinumab is a monoclonal antibody that neutralizes IL-1β, preventing IL-1R activation.
Our team would be happy to answer any questions about IL-1β or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
Inflammasome – A Therapeutic Target for Multiple Diseases
An overview of inflammasomes, including their mechanisms of action, roles in diseases, and targeting for drug development.
What is NLRP3?
An overview of NLRP3 inflammasome activation triggers, disease associations, and therapeutic targeting strategies.
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.
Lysosome Dysfunction in Microglia & Astrocytes
An overview of lysosomal dysfunction in microglia & astrocytes, and its role in neurodegenerative diseases.
Mitochondrial Dysfunction in Microglia & Astrocytes
The role of mitochondrial dysfunction in microglia and astrocytes in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.