Biospective's AAV A53T α-synuclein mouse model of Parkinson’s disease is a gene delivery–based preclinical model that induces rapid α-synuclein aggregation, dopaminergic neurodegeneration, neuroinflammation, and motor deficits. Biospective offers this extensively characterized animal model as part of its global preclinical CRO services, supporting efficacy testing, biodistribution, mechanism-of-action, and target engagement studies using translational endpoints including CSF neurofilament light chain (NfL), MRI-based brain atrophy, and quantitative multiplex immunofluorescence.
In this robust mouse model, adeno-associated virus (AAV) vectors overexpressing the human A53T-mutant α-synuclein (SNCA) gene are delivered into the substantia nigra pars compacta via stereotaxic injection. The resulting model demonstrates α-synuclein aggregation, dopaminergic neuron loss, neuroinflammation (activated microglia and astrocytes), and motor deficits reminiscent of human Parkinson’s disease.
Biospective has extensively characterized this model and leverages it as an ideal platform for preclinical drug development, supporting high-throughput efficacy testing, biodistribution analysis, mechanism-of-action studies, and target engagement evaluation for novel Parkinson’s therapeutics. As a specialized neuroscience CRO, we provide fully integrated, end-to-end services – from surgical model induction and in vivo imaging to biomarker assays and quantitative pathology – delivering high-quality, decision-ready data for biotech and pharmaceutical clients worldwide.
Overview of the AAV A53T α-Synuclein Model of Parkinson's Disease
A rapidly inducible alpha-synuclein animal model of Parkinson's disease optimized for preclinical drug development.
In this model, adeno-associated virus (AAV) vectors encoding human A53T-mutant α-synuclein (SNCA) undergo stereotaxic injection into the midbrain substantia nigra of young adult C57BL/6 mice. This targeted intra-nigral delivery drives high levels of toxic α-synuclein expression, triggering a cascade of Parkinson’s-like pathology. The AAV-A53T model faithfully recapitulates key hallmarks of PD, including:
-
Dopaminergic neuron loss: Substantial degeneration of dopamine-producing neurons in the substantia nigra pars compacta, with corresponding loss of dopaminergic nerve fibers in the striatum.
-
α-Synuclein aggregation: Accumulation of pathogenic α-synuclein (especially phosphorylated pSyn Ser129), forming Lewy body–like intracellular inclusions in affected brain regions.
-
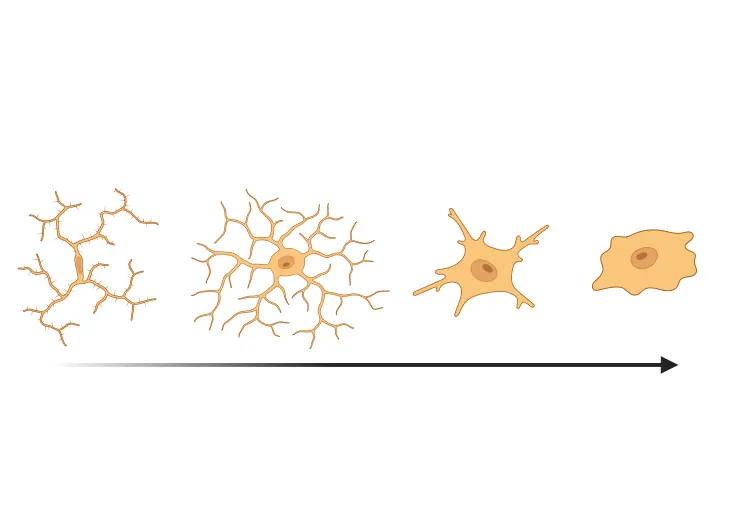
Robust neuroinflammation: Pronounced activation of microglia and reactive astrocytes in areas of α-synuclein pathology, mirroring the neuroinflammatory response observed in Parkinson’s disease.
-
Motor function deficits: Significant motor impairments (e.g. limb use asymmetry, coordination and balance deficits) arising from nigrostriatal neurodegeneration, reminiscent of Parkinsonian motor symptoms.
By reproducing these pathological features, the AAV A53T model provides a disease-relevant platform to evaluate therapeutic interventions under conditions that mirror the clinical hallmarks of human Parkinson’s disease.
Importantly, the AAV-Syn model yields rapid and robust disease manifestation on an accelerated timeline. Traditional transgenic α-synuclein mouse models often require 6–12 months to develop mild phenotypes and may not exhibit strong nigrostriatal degeneration. In contrast, AAV-A53T mice develop measurable neuron loss and motor deficits within weeks of vector injection. Typically, by ~5 weeks post-injection, significant dopaminergic neuron loss in the substantia nigra is observed alongside clear behavioral deficits. This fast onset enables shorter studies and faster go/no-go decisions in preclinical programs without sacrificing biological relevance. The compressed in vivo timeline makes the AAV-synuclein model highly suited for high-throughput efficacy screening and proof-of-concept studies in Parkinson’s research.
Biospective's AAV-Synuclein Model Expertise and Services
Biospective is a global neuroscience CRO with deep expertise in Parkinson’s disease animal models – particularly the AAV A53T α-synuclein model, which is a core part of our service portfolio.
We have spent over a decade developing and executing studies in α-synuclein models, giving us unrivaled insight into their nuances and optimal use in drug development. Our experienced team works as an extension of your own, ensuring rigorous study design and translational relevance at every step.
Some key advantages of partnering with Biospective for AAV-synuclein studies include:
-
Extensive Experience & Characterization: 10+ years of continuous experience with preclinical α-synuclein models. We have extensively characterized the AAV-A53T mouse model through numerous studies, generating datasets that inform best practices and enhance reproducibility. This track record underscores our unique expertise with this Parkinson’s model.
-
Optimized AAV Vectors & Rapid Study Start: We utilize high-titer, validated AAV vectors encoding human A53T α-synuclein to ensure robust, consistent model induction. Biospective maintains ready access to these viral vectors in-house, enabling fast study start-up without delays. Precise stereotaxic injection techniques and optimized dosing result in reliable pathology, and our on-demand vector supply accelerates project timelines.
-
End-to-End Preclinical Services: Biospective provides fully integrated services from initial study design through execution and data analysis. Our capabilities cover all aspects of the project, including surgical model induction (skilled unilateral AAV injections into the substantia nigra), comprehensive in-life assessments (behavioral testing, motor function assays, etc.), in vivo neuroimaging (MRI, PET) for longitudinal monitoring, biofluid collection (CSF, blood) for biomarker analysis, and post-mortem histopathology (immunohistochemistry and multiplex immunofluorescence). This one-stop approach ensures consistency, quality control, and efficient timelines.
- Translational Biomarkers & Readouts: We incorporate clinically relevant biomarkers that bridge preclinical findings to clinical outcomes. For example, we measure neurofilament light chain (NfL) levels in CSF as a biomarker of neurodegeneration (analogous to patient studies), and we perform MRI brain imaging to quantify neurodegenerative atrophy. We also conduct quantitative IHC (e.g. p-α-syn, TH for dopaminergic neurons, Iba1 for microglia) and multiplex immunofluorescence to assess pathology and neuroinflammation in tissue. These advanced readouts enhance the translatability of study results to human trials.
-
Global Collaboration & Flexibility: As a global preclinical CRO, we serve biotech and pharmaceutical clients worldwide and tailor each AAV-synuclein study to your therapeutic strategy. Our scientists collaborate closely with your team to customize protocols – from adjusting injection parameters (e.g. targeting specific brain regions, unilateral vs. bilateral injections) to incorporating novel endpoints or treatment paradigms. We offer flexibility to meet program-specific needs while maintaining scientific rigor, reproducibility, and transparent communication throughout the partnership.
By leveraging these strengths, Biospective empowers your team to efficiently generate decision-quality data in the AAV α-synuclein model. We pride ourselves on fast project initiation, meticulous data analysis, and supporting our clients through all preclinical phases of Parkinson’s therapy development.
AAV-Syn Model Generation & Study Timeline
Our expert team employs state-of-the-art, precise stereotaxic surgery techniques to generate the AAV-A53T model with minimal study start-up times.
We inject high-titer AAV vectors (e.g. AAV1/2 carrying the human A53T SNCA gene) unilaterally into the midbrain substantia nigra (SN) region of ~12-week-old C57BL/6 mice. In these surgeries, we utilize digital stereotaxic systems with automated microinjectors to ensure accurate targeting and controlled viral delivery. This refined methodology yields consistent α-synuclein overexpression localized to the nigrostriatal pathway.
Following injection, Parkinsonian neuropathology evolves rapidly. Animals begin to exhibit motor deficits (e.g. limb use asymmetry) within a few weeks post-injection, coinciding with ongoing dopaminergic neuron loss and protein aggregation in the affected brain regions. By ~5 weeks post-AAV injection, robust disease endpoints can be captured.
An illustration of the process of AAV-A53T Synuclein model generation.
This abbreviated timeline is a significant advantage as it enables quicker iteration and rapid data readouts, in contrast to transgenic models that might require 6–12+ months to develop endpoints. The fast onset and severity of pathology in the AAV-A53T model, therefore, provide an efficient system for testing therapeutic efficacy and mechanistic hypotheses in preclinical Parkinson’s disease programs. Biospective can initiate studies with this model on-demand, thanks to our in-house capabilities (including ready access to viral vector stock), ensuring minimal startup time for your project.
Validated Endpoints & Translational Biomarkers
Biospective has implemented a suite of validated endpoints and Parkinson's disease relevant biomarkers to enable clinical advancement of therapeutic programs.
To fully characterize the AAV-A53T model and assess treatment outcomes, Biospective has validated a broad spectrum of endpoints – encompassing behavioral assays, neuroimaging, fluid biomarkers, and histopathology. This comprehensive approach yields robust, quantitative readouts for both efficacy and mechanism-of-action in preclinical studies. Key validated endpoints in our AAV α-synuclein model include:
Behavioral & Functional Endpoints
-
Hindlimb Clasping Test: A sensitive indicator of neurodegeneration (brainstem/spinal reflex integrity) often observed as disease progresses.
-
Tail Suspension Swing Test: Assesses lateral bias in movement during a tail suspension, indicating unilateral motor deficits resulting from nigrostriatal damage.
-
Cylinder Test: Measures forelimb use asymmetry during rearing and exploratory behavior. Reduced use of the contralateral paw reflects motor impairment on the side of the lesion.
-
Rotarod Test: Evaluates motor coordination and balance. Declining performance on the rotarod apparatus indicates motor function deficits typical of Parkinsonian impairment.
Imaging, Fluid & Tissue Biomarkers
-
MRI Brain Atrophy: In vivo magnetic resonance imaging to quantify regional brain volume loss (neurodegeneration) over time. Progressive MRI-detected atrophy in the midbrain and connected structures serves as a translational endpoint paralleling human PD.
-
CSF Neurofilament Light Chain (NfL): A fluid biomarker of axonal damage and neurodegeneration, measured in cerebrospinal fluid (and optionally blood plasma). Elevated NfL levels indicate ongoing neuronal injury; this biomarker is also used in clinical trials, making it a valuable bridge between preclinical and clinical results.
-
Quantitative Histopathology (IHC/mIF): High-resolution tissue analyses to quantify PD-related pathology. We perform immunohistochemistry (IHC) and multiplex immunofluorescence for markers such as phosphorylated α-synuclein inclusions (pSyn Ser129), dopaminergic neurons (tyrosine hydroxylase, TH), activated microglia (Iba1), and astrocytes (GFAP). Digital image analysis of these stained tissues provides quantitative measures of Lewy-like aggregates, neuronal loss, and neuroinflammation in the brain.
These endpoints span multiple domains – behavioral, imaging, biochemical, and histological – providing complementary measures of disease severity and therapeutic impact. Notably, the inclusion of translational biomarkers like MRI volumetry and NfL helps bridge preclinical findings to the clinic. Neurofilament light (NfL) is a well-established marker of neurodegeneration: when neurons are damaged, NfL is released into CSF and blood, serving as a sensitive indicator of axonal injury and neurodegeneration. In clinical studies, elevated NfL levels correlate with disease progression in various neurological disorders, including Parkinson’s disease (see our Resource - Neurofilament Light Chain in Parkinson's Disease Models). Within our AAV-A53T studies, we observe a similar pattern – as dopaminergic neurons degenerate, CSF & plasma NfL levels rise in parallel with MRI-detected brain atrophy. This mirrored trend underscores the predictive, translational value of our readouts. By tracking such biomarkers longitudinally in vivo, we can quantitatively monitor disease progression and detect therapeutic effects in a way that is directly relatable to patient outcomes.
In addition to these outcome measures, Biospective distinguishes itself by offering seamless end-to-end integration of all study components. We handle every aspect of the experiment – from viral vector administration, longitudinal behavioral testing, and in vivo MRI/PET imaging to biofluid collection and post-mortem tissue analysis. Our scientific team employs advanced analytics (including automated image analysis for dopaminergic terminal density and AI-driven cell morphology classification) to extract rich datasets from the model. (See our Presentation - Microglial Activation in an α-Synuclein Mouse Model of Parkinson's Disease). All data are rigorously analyzed and integrated into an interpretable report, allowing you to make informed decisions on your therapeutic candidate’s performance.
Interactive Microscopy Images
Use the Image Viewer below to navigate through high-resolution microscopy images via the left-hand panel or the on-screen arrows. You can pan around the images with your mouse, and zoom in/out using the scroll wheel or the +/- controls. The Control Panel (top-right) allows toggling of image channels and segmentation overlays. For the best experience, we recommend switching to full-screen mode.
Multiplex immunofluorescence tissue section that demonstrates phosphorylated α-synuclein aggregates, activated microglia, and dopaminergic neuron loss ipsilateral to AAV-Syn injection into the substantia nigra (left hemisphere) in a C57BL/6 mouse.
Click to copy link
This comprehensive capability means that whether you aim to measure drug biodistribution, target engagement (e.g. α-syn clearance), synaptic integrity, and/or neuroinflammatory modulation, our team can incorporate the appropriate assays and analyses into the study design. All data are rigorously analyzed and integrated into an interpretable package, allowing you to make informed decisions on your therapeutic candidate’s performance.
Leverage Biospective's AAV-Syn Model for Parkinson’s Drug Development
By partnering with Biospective for your Parkinson’s disease research, you gain access to an internationally recognized team of neurobiology experts and a deeply characterized preclinical model that can accelerate your drug development pipeline.
We have extensive experience executing studies in the AAV A53T α-synuclein model – from exploratory proof-of-concept efficacy studies to detailed mechanistic investigations – across a range of therapeutic modalities (small molecules, biologics, antibodies, gene therapies, antisense oligonucleotides, etc.). Our commitment to scientific rigor and translational relevance is reflected in the quality of our data and our continuous innovation in model validation. As a full-service CRO, we integrate study design, execution, analysis, and reporting, ensuring that your Parkinson’s therapy candidates are evaluated with the highest level of expertise and care.
Contact us to discuss how our AAV α-synuclein mouse model and end-to-end preclinical services can support your Parkinson’s disease drug development program.
Discover more of our Parkinson's Disease Models
Related Content
Up-to-date information on Parkinson's Disease and best practices related to the evaluation of therapeutic agents in PD animal models.
AAV α-Synuclein Models for Parkinson's Disease Drug Development
Overview of adeno-associated virus (AAV) induced α-synuclein expression in mouse & rat models for use in preclinical studies of disease-modifying therapeutics.
Neurofilament Light Chain in Parkinson's Disease Models
How neurofilament light chain (NfL; NF-L) levels can be used as blood (plasma; serum) & CSF biomarkers in Parkinson's disease mouse and rat models.
Microglial Activation in an α-Synuclein PFF Mouse Model
We have quantified microglial activation, based on morphology, in an α-synuclein preformed fibril (PFF) seeding & spreading mouse model of Parkinson’s disease.