What is NLRP3?
NLRP3 (NOD-like receptor pyrin domain-containing protein 3), also referred to as cryopyrin, is a cytosolic protein that acts as a sensor for harmful or stress-related signals in the innate immune system. It detects various danger signals, such as cellular damage or stress and initiates the formation of a larger protein complex, called the “inflammasome”.
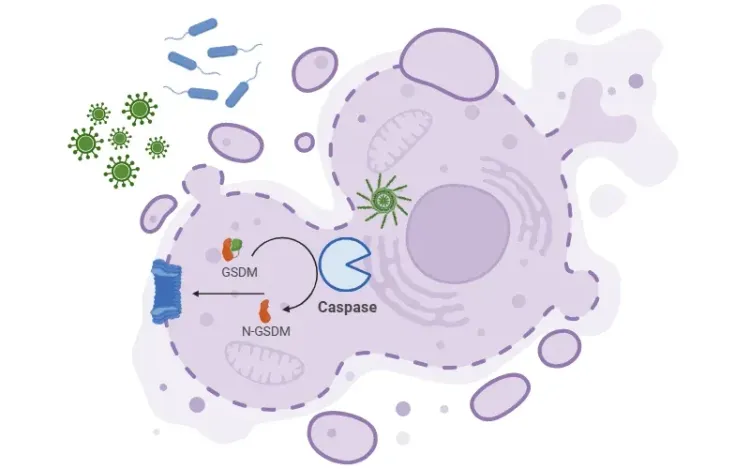
This process begins when NLRP3 recruits ASC (an adaptor molecule) and pro-caspase-1. Together, they form the inflammasome, and their assembly leads to the activation of caspase-1, a key enzyme in the inflammatory response. Caspase-1 then cleaves pro-inflammatory cytokines like IL-1β and IL-18 into their active forms, which are released from the cell and promote inflammation. NLRP3 activation also can lead to the cleavage of gasdermin D (GSDMD) by caspase-1, triggering pyroptosis, a form of programmed cell death that further amplifies inflammation.
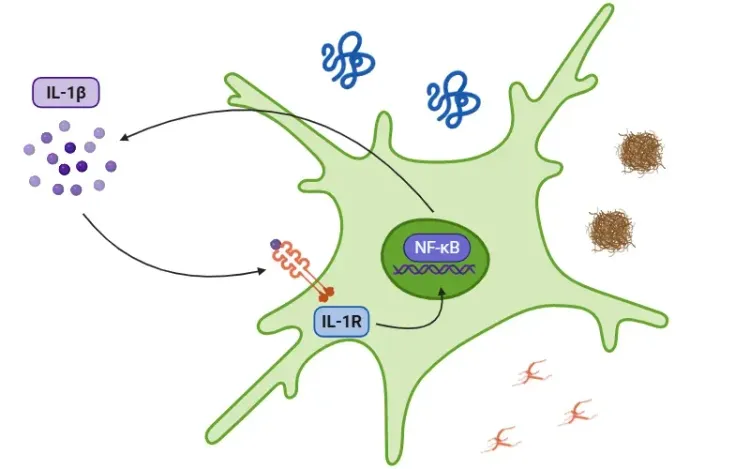
For more on the role of IL-1β, see: What is IL-1β (Interleukin-1 Beta)? & Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
See also: What is Pyroptosis?
NLRP3 is activated by PAMPs (Pathogen-Associated Molecular Patterns) and DAMPs (Damage-Associated Molecular Patterns), which are signals from microbes or injured cells.
Examples of NLRP3-activating stimuli:
- PAMPs: Bacterial toxins or viral RNA
- DAMPs: Extracellular ATP, uric acid crystals, or protein aggregates like amyloid-beta (Aβ)
While the NLRP3 inflammasome is vital for host defense, its persistent activation can drive chronic inflammation and contribute to tissue damage.
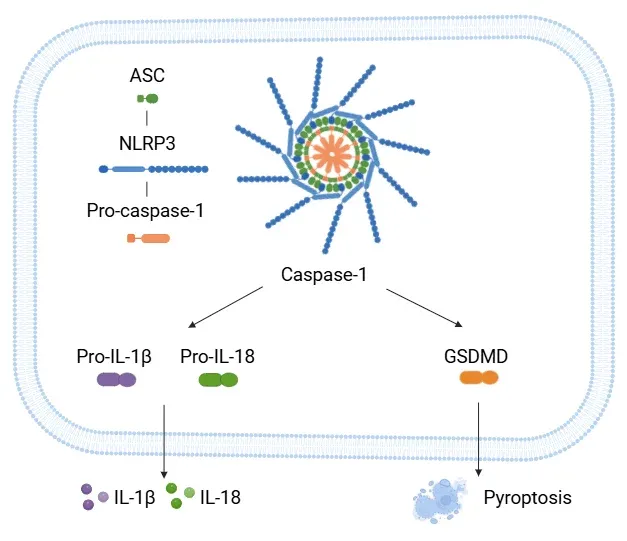
NLPR3 Activation Pathway.
The NLRP3 inflammasome is composed of NLRP3, ASC, and pro-caspase-1. Upon activation, the complex promotes the cleavage of pro-caspase-1 into active caspase-1, which in turn processes pro-IL-1β and pro-IL-18 into their active cytokine forms. Caspase-1 also cleaves GSDMD, resulting in pyroptosis.
NLRP3 is the most studied member of the inflammasome family, but it is part of a larger network that includes other inflammasome-forming sensors, which respond to different types of cellular stress:
- NLRP1 has been linked to autoimmune diseases and skin inflammation
- AIM2 detects cytosolic DNA and is implicated in inflammatory diseases
- NLRC4 responds to bacterial flagellin and is involved in gastrointestinal inflammation.
Understanding the interplay among these inflammasomes is essential, as many diseases involve more than one pathway.
What is the role of NLRP3 in disease?
Inflammasomes contribute to a wide range of diseases affecting both the central nervous system (CNS) and peripheral organs and tissue. In the CNS, their chronic activation is increasingly recognized as a driving force behind neurodegeneration.
In Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), misfolded proteins like Aβ, tau, α-synuclein, and TDP-43 act as DAMPs, activating NLRP3 (Holbrook, 2021; Singh, 2023; Wang, 2024). This activation leads to sustained inflammation and neuronal injury. Knockout studies in mice lacking NLRP3 components show reduced pathology and slower disease progression, supporting the utility of NLRP3 inhibition as a therapeutic intervention for neurodegenerative diseases (Heneka, 2013; Qiao, 2017; Ising, 2019; Lee, 2019).
For a review on the role of the NLRP3 inflammasome in neurodegenerative diseases, see: NLRP3 Inflammasome and Neurodegenerative Disease,
Other inflammasomes implicated in CNS diseases:
- AIM2: Upregulated in AD and PD (Yang, 2024)
- NLRP1: Associated with pyroptotic neuron death in AD models (Singh, 2023)
Outside the CNS, NLRP3 contributes to several chronic inflammatory and metabolic diseases. In type 2 diabetes, NLRP3 activation is associated with insulin resistance, particularly in adipose tissue (Nițulescu, 2023). In cardiovascular disease, NLRP3 activation promotes plaque formation in atherosclerosis (Tanase, 2023). In gout, uric acid is the trigger (Kim, 2022). NLRP3 also plays a role in the inflammatory response to infectious diseases, including COVID-19 and influenza (Li, 2024).
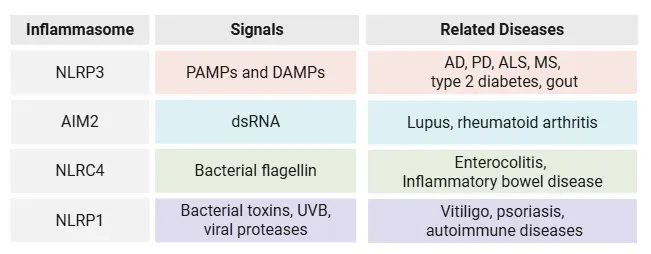
Table highlighting the diversity of inflammasome sensors, the types of external stimuli that activate them, and their roles in various inflammatory, neurodegenerative, autoimmune, and metabolic conditions.
How do therapies target the NLRP3 inflammasome to treat disease?
Given its role in so many diseases, the NLRP3 inflammasome is an important therapeutic target. Researchers are actively exploring drugs that can safely suppress NLRP3 activity without compromising immune defense (Blevins, 2022).
Therapeutic strategies include:
- Direct inhibitors:
- MCC950 is a selective NLRP3 inhibitor that reduces inflammation in animal models of neurodegenerative diseases (Gordon, 2018; Blevins, 2022; Grotemeyer, 2023)
- Oral agents, like Dapansutrile (OLT117) and Usnoflast (ZYIL1), are in clinical development
- Downstream blockers (Wang, 2024):
- VX-765 selectively inhibits caspase-1
- Disulfiram blocks gasdermin D to reduce inflammation and cell death
- IC100, an ASC inhibitor, aims to prevent inflammasome assembly
Some diseases involve multiple inflammasomes working in parallel. This scenario has led to interest in dual or broad-spectrum inhibitors, particularly for complex conditions like ALS (Clénet, 2023).
Despite progress, key challenges remain:
- Delivering therapeutic agents across the blood-brain barrier is a major hurdle for neurological diseases
- Long-term immune suppression may increase infection risk
- The redundancy of inflammasome pathways means that inhibiting NLRP3 may not be effective in all disease contexts
Our team would be happy to answer any questions about NLRP3 or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
What is Pyroptosis? | A Drug Development Perspective
An overview of pyroptosis, its role in various diseases, and therapeutic strategies related to pyroptosis pathways.
Inflammasome – A Therapeutic Target for Multiple Diseases
An overview of inflammasomes, including their mechanisms of action, roles in diseases, and targeting for drug development.
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.
What Is IL-1β (IL-1b)? Function, Signaling, and Biological Role
An overview of IL-1β, including its signaling pathways, involvement in disease mechanisms, and potential therapeutic targets.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
Lysosome Dysfunction in Microglia & Astrocytes
An overview of lysosomal dysfunction in microglia & astrocytes, and its role in neurodegenerative diseases.
Mitochondrial Dysfunction in Microglia & Astrocytes
The role of mitochondrial dysfunction in microglia and astrocytes in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.