This resource describes:
What is an inflammasome?
An inflammasome is a cytosolic multiprotein complex that plays a critical role in the innate immune response by detecting pathogens and cellular stress signals. These complexes typically form in response to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and serve as central regulators of inflammation.
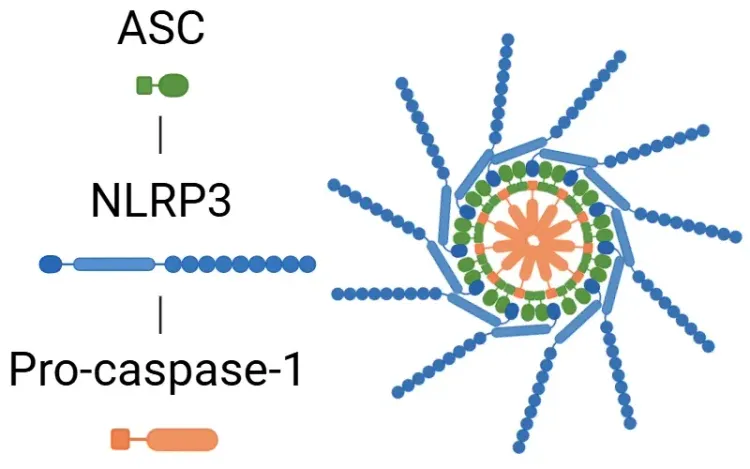
Structurally, inflammasomes consist of a sensor protein – such as a NOD-like receptor (NLR) like NLRP1, NLRP3, and NLRC4, or the absent in melanoma 2 (AIM2) receptor. Upon activation, these sensors recruit the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) and the effector enzyme pro-caspase-1, forming the active inflammasome complex. However, not all inflammasomes follow the same assembly process. For example, the CARD domain of NLRP1 can directly interact with pro-caspase-1, bypassing the need for ASC (Lu, 2023).
Inflammasome activation generally involves a two-step process: priming and activation.
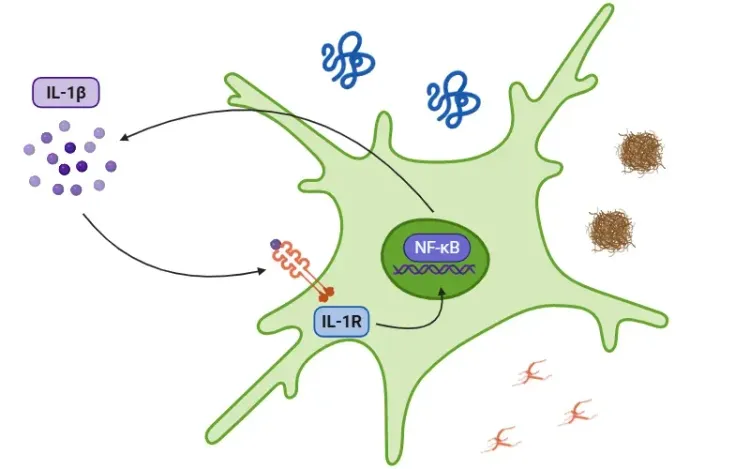
- Priming typically involves signaling through pathways such as NF-κB, which upregulate the expression of inflammasome components, including pro-IL-1β and pro-IL-18 (Lu, 2023; Liao, 2025).
- Activation occurs in response to a secondary signal, prompting assembly of the inflammasome complex and activation of caspase-1 (Liu, 2018).
Among all known inflammasomes, NLRP3 is the most extensively studied due to its broad range of activation triggers, including ATP, amyloid-β (Aβ) aggregates, and crystalline substances like uric acid. Additional triggers include ion flux, lysosomal damage, and mitochondrial dysfunction.
See: What is NLRP3?
Other inflammasomes are activated by more specific triggers:
- AIM2 responds to cytosolic DNA
- NLRC4 is activated by bacterial flagellin
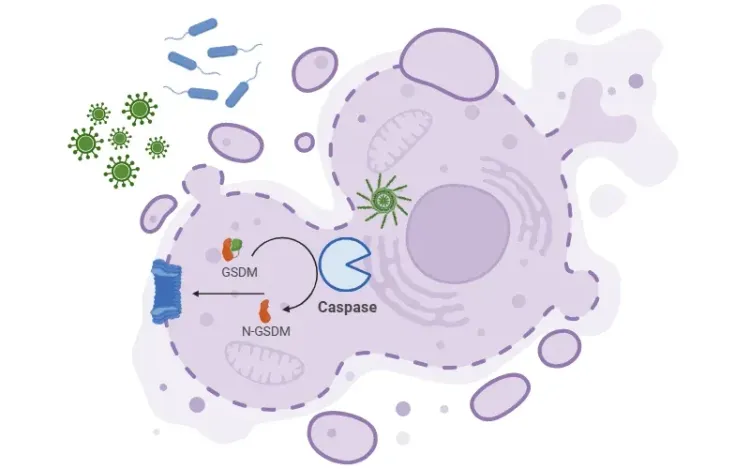
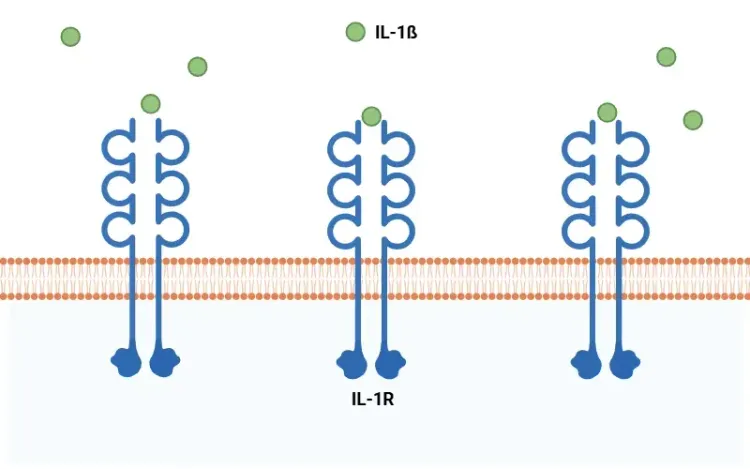
Once activated, caspase-1 cleaves the precursor cytokines pro-IL-1β and pro-IL-18 into their mature forms – IL-1β and IL-18 – which are secreted and propagate inflammation. Caspase-1 also induces pyroptosis, a form of programmed, pro-inflammatory cell death.
See: What is IL-1β? and What is Pyroptosis?
While inflammasomes are vital for host defense, chronic or dysregulated activation is associated with a wide range of diseases, including autoimmune disorders, neurodegenerative diseases, and metabolic syndromes (Guo, 2015).
In summary, inflammasomes are essential regulators of innate immunity. However, their activity must be tightly controlled to prevent pathological inflammation.
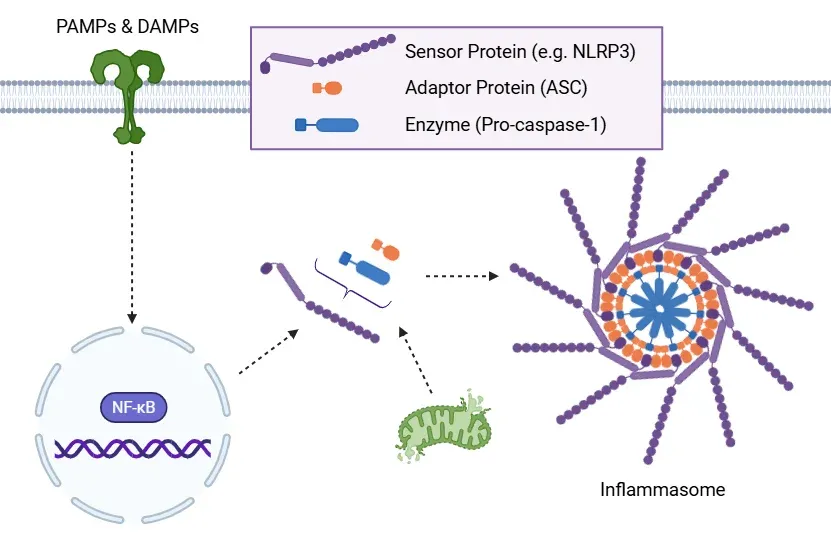
Canonical Inflammasome Activation Pathway.
Schematic illustration of inflammasome activation. During priming, PAMPs and DAMPs activate NF-κB signaling, increasing the expression of inflammasome components. Subsequent activation – triggered by factors such as mitochondrial destabilization – leads to inflammasome assembly via recruitment of ASC and pro-caspase-1.
What is the role of inflammasomes in disease?
Although Inflammasomes are critical for host defense, dysregulated inflammasome activity – whether excessive, prolonged, or insufficient – can contribute to the development of numerous acute and chronic diseases by promoting tissue damage, chronic inflammation, and immune dysregulation.
Key Roles in Disease Pathogenesis
- Autoinflammatory and Autoimmune Diseases
- Mutations in NLRP3 are liked to hereditary autoinflammatory syndromes like familial cold autoinflammatory syndrome (FCAS) and Muckle-Wells syndrome (MWS) (Kuemmerle-Deschner, 2011; Chen, 2022).
- Dysregulation of NLRP1, NLRP3 and AIM2 is associated with autoimmune diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis (Yi, 2018).
- Cancer
- The AIM2 inflammasome has a dual role in cancer, acting as both a tumor suppressor and tumor promoter, depending on the context. These roles have been observed in cancers such as colorectal, liver, and gastric cancer (Alanazi, 2024).
- Cardiovascular Diseases
- In atherosclerosis, cholesterol crystals activate NLRP3 in macrophages, promoting plaque inflammation (Yalcinkaya, 2025).
- NLRP3, NLRC4, and AIM2 have been implicated in the progression of heart failure (Wu, 2021).
- Metabolic Disorders
- In type 2 diabetes, NLRP3 activation in adipose tissue and pancreatic islets contributes to insulin resistance and β-cell dysfunction (Lu, 2023).
- Obesity leads to chronic low-grade inflammation, partly due to NLRP3 activation by fatty acids and mitochondrial reactive oxygen species (ROS) (Ahechu, 2018).
- Gout is caused by monosodium urate crystals, which are potent activators of the NLRP3 inflammasome (Kim, 2022).
- Neurodegeneration Diseases
- Chronic NLRP3 activation contributes to Alzheimer’s disease (AD) by contributing to amyloid-β pathology, tau hyperphosphorylation, and neuronal damage (Li, 2025).
- Emerging studies also implicate inflammasomes to Parkinsons’s disease (PD), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS).
See: NLRP3 Inflammasome and Neurodegenerative Diseases for a detailed discussion.
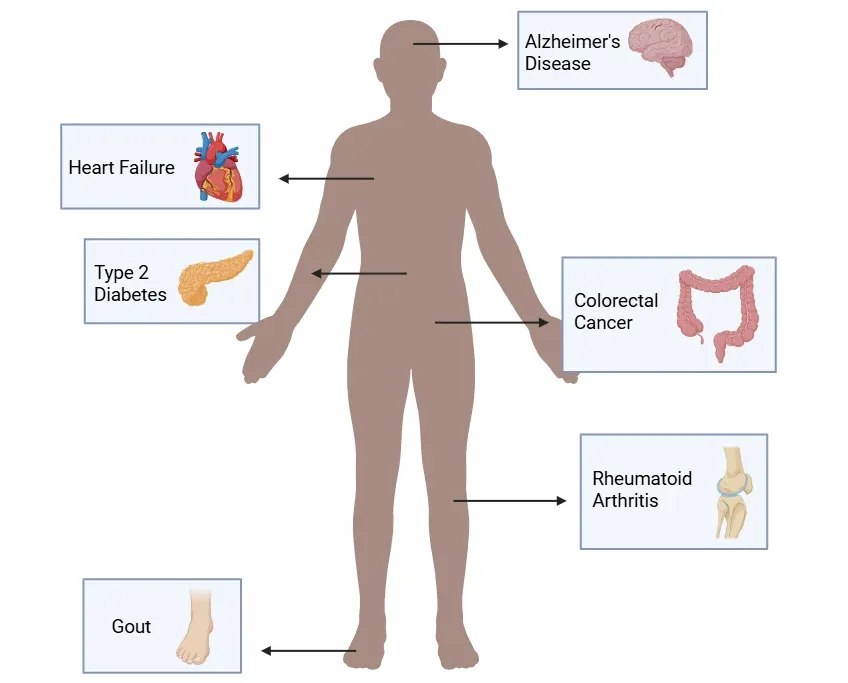
Representative Diseases Associated with Inflammasome Activation.
Inflammasome dysregulation is linked to a wide spectrum of diseases, including neurodegenerative, cardiovascular, autoimmune, and metabolic conditions. The figure highlights key examples, though many other diseases also involve inflammasome pathways.
How do therapies target inflammasomes?
Therapies that target inflammasomes aim to suppress pathological inflammation by disrupting key points in the inflammasome signaling cascade. Most current strategies focus on NLRP3, given its central role in many inflammatory diseases.
Direct NLRP3 Inhibition
- MCC950 is a selective small-molecule inhibitor that blocks NLRP3 activation and prevents the release of IL-18 and IL-1β. It has shown efficacy in multiple preclinical disease models (Dempsey, 2017; Gordon, 2018; Gao, 2019).
- Other agents, such as dapansutrile (OLT1177®), are under investigation, with several in early-stage clinical trials (NCT06047262; NCT05658575).
Caspase-1 Inhibition
- VX-765, a caspase-1 inhibitor, has demonstrated effectiveness in reducing cytokine production and pyroptosis both in vitro and in vivo (McKenzie, 2018; Flores, 2020).
IL-1β Pathway Blockade
Blocking IL-1β signaling downstream of inflammasome activation is a validated therapeutic approach:
- Anakinra (IL-1 receptor antagonist)
- Canakinumab (monoclonal antibody targeting IL-1β)
- Rilonacept (soluble decoy receptor)
These agents are approved for various autoinflammatory conditions and are being tested for broader indications, including cardiovascular and neurodegenerative diseases (Wu, 2021; Melchiorri, 2023).
Targeting Pyroptosis and Gasdermin D
Emerging research is exploring gasdermin D, the key executor of pyroptosis. By inhibiting gasdermin D, it may be possible to block inflammatory cell death while preserving cytokine signaling – offering a targeted and potentially safer therapeutic strategy (Dai, 2023).
In summary, inflammasome-targeted therapies hold great promise for treating a wide range of inflammatory and autoimmune diseases. However, due to their essential role in immune defense, these therapies must be carefully modulated to avoid impairing host immunity.
Our team would be happy to answer any questions about Inflammasomes or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
What is Pyroptosis? | A Drug Development Perspective
An overview of pyroptosis, its role in various diseases, and therapeutic strategies related to pyroptosis pathways.
What is NLRP3?
An overview of NLRP3 inflammasome activation triggers, disease associations, and therapeutic targeting strategies.
What Is IL-1β (IL-1b)? Function, Signaling, and Biological Role
An overview of IL-1β, including its signaling pathways, involvement in disease mechanisms, and potential therapeutic targets.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.
Mitochondrial Dysfunction in Microglia & Astrocytes
The role of mitochondrial dysfunction in microglia and astrocytes in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.