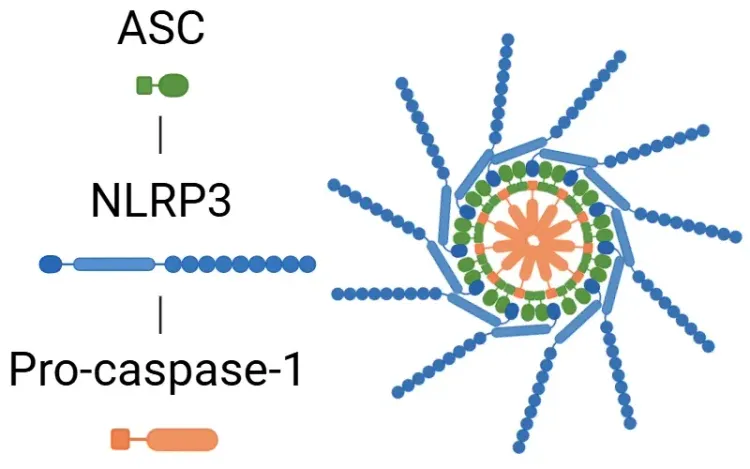
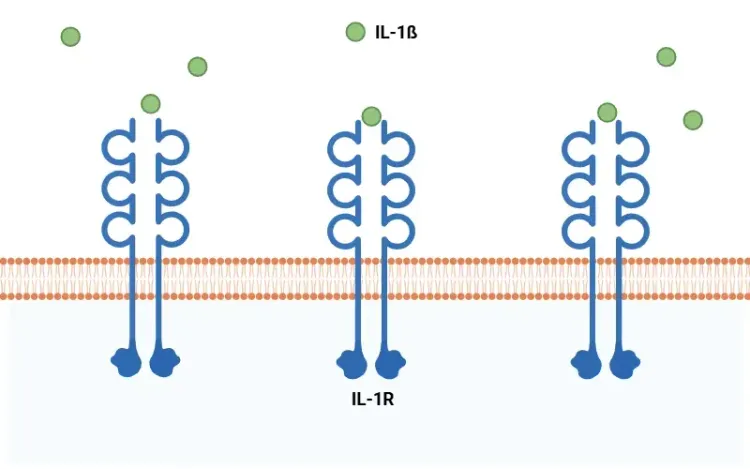
What is IL-1β?
Interleukin-1 Beta (IL-1β or IL-1beta) is a pro-inflammatory cytokine from the IL-1 superfamily, primarily produced by monocytes, macrophages, and neutrophils, with high concentrations in various tissues. It plays a vital role in:
- Host defense
- Inflammation
- Fever induction
- Immune cell activation
- Brain function
Under physiological conditions in the central nervous system (CNS), IL-1β promotes:
- Neuronal proliferation
- Differentiation
- Apoptosis
- Long-term potentiation
For a detailed summary of IL-1β, see: What is IL-1β?
IL-1β is secreted in response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), activating signaling pathways that promote inflammation through mechanisms involving the NLRP3 inflammasome and various kinases, including TNF-α and IL-18 (Lopez-Castejon, 2011; Kaneko, 2019).
Elevated IL-1β levels can lead to excessive inflammation, contributing to diseases such as gout, osteoarthritis, and cancer by promoting inflammation and helping tumor cells evade the immune system. In neurodegenerative diseases, IL-1β is involved in neuroinflammation, particularly through the NLRP3 inflammasome, with implications for neurodegenerative diseases such as AD and PD.
See: What is an Inflammasome? & NLRP3 Inflammasome and Neurodegenerative Diseases
What is the role of IL-1β in AD, PD, and ALS?
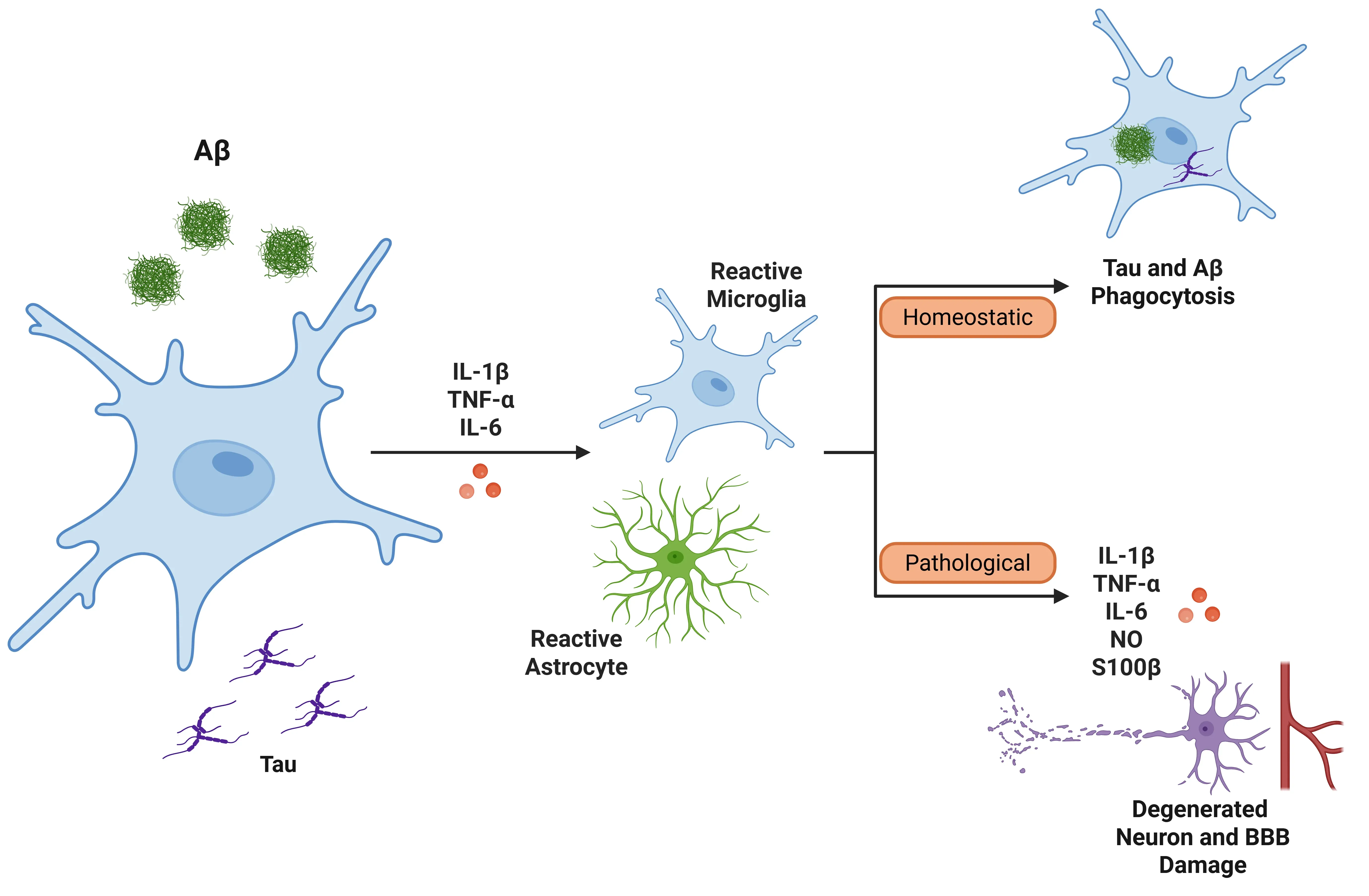
Microglia and astrocytes are two important glial cell types that help maintain homeostasis in the CNS. Both of these cells play vital roles in the brain's immune response and are key contributors to neuroinflammation, often present in neurodegenerative diseases:
- Microglia can secrete IL-1β in pro-inflammatory conditions. Specifically, disease-associated microglia (DAMs) can have both protective and harmful effects in neurodegenerative diseases. Initially, they can protect against disease by clearing neurotoxic aggregates through phagocytosis. However, DAMs can also release pro-inflammatory cytokines, such as IL-1β and TNF-α, which can worsen chronic inflammation and neurodegeneration. DAM-like microglia have been identified in various neurodegenerative diseases, including tauopathies and AD, PD, and ALS.
- Astrocytes can also secrete IL-1β under pro-inflammatory conditions. Some reactive astrocytes can form a glial scar as a protective mechanism, serving as a barrier to prevent further damage and the spread of infection. However, reactive astrocytes can also produce pro-inflammatory cytokines that exacerbate neuronal injury.
See: Microglia, Astrocytes & Tau in Neurodegenerative Diseases & Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease.
Alzheimer's Disease (AD)
AD is recognized as the most prevalent form of dementia, characterized by the accumulation of proteins in the brain, which manifests as amyloid plaques and neurofibrillary tau tangles. A key player in the pathology of AD is IL-1β, which exhibits a complex role in regulating both amyloid-beta and tau pathology. Its function can be both beneficial and detrimental (Shaftel, 2008; Matousek, 2012; Boraschi, 2023). On the negative side, IL-1β is produced by the NLRP3 inflammasome, leading to neuroinflammation and neuronal damage associated with AD (Lopez-Rodriguez, 2021; Boraschi, 2023).
Other cytokines also participate in the inflammatory processes underlying AD. Levels of IL-6, IL-12, IL-18, TNF-α, and TGF-β are significantly elevated in the peripheral blood of individuals with AD (Xu, 2024). Genetic factors also contribute to the development of the disease. Specific polymorphisms in the IL-1 gene that are associated with an increased risk of AD include (Mrak, 2001):
- IL-1A 2 (-889): This variant is linked to higher expression levels of IL-1α.
- IL-1B 2 (exon 5): This variant is linked to higher expression levels of IL-1β.
IL-1β can activate microglia and astrocytes in the context of AD (Ghosh, 2013). When IL-1β is released, it induces the expression of other pro-inflammatory cytokines, such as TNF-α and IL-6, further amplifying the inflammatory response.
- Microglia: Microglia can initially aid against AD by clearing amyloid-beta; chronic activation can overwhelm this system, resulting in neuroinflammation. This activation leads to a feedback loop where microglia release more IL-1β and additional inflammatory mediators, ultimately compromising amyloid-beta clearance and damaging the blood-brain barrier (BBB) (Shaftel, 2008; Valiukas, 2025).
- Astrocytes: Astrocytes can produce exaggerated responses to IL-1β when primed by amyloid-beta, contributing further to inflammation. The release of nitric oxide induced by IL-1β may also lead to neurotoxicity. , particularly those overexpressing IL-1β, can release S100β, a protein implicated in neuronal damage and the exacerbation of inflammatory processes (Mrak, 2000; Sama, 2008).
IL-1β is involved in tau pathology, as it stimulates the hyperphosphorylation of tau protein through microglial activation and MAPK signalling pathways, which may disrupt microtubule stability and contribute to neurofibrillary tangles (Griffin, 2006; Shaftel, 2008; Ghosh, 2013). Additionally, IL-1β is linked to the production and deposition of amyloid-beta. Inflammatory cytokines, including IL-1β, IL-6, and TGF-β, accumulate around amyloid plaques in the brains of individuals with AD (Italiani, 2018). Overexpression of IL-1β correlates with increased plaque formation and the growth of dystrophic neurites (Mrak, 2001).
Interestingly, despite its pro-inflammatory role, IL-1β may also engage in protective mechanisms against AD. In a transgenic mouse model of AD designed for IL-1β overexpression, prolonged neuroinflammation led to activated astrocytes and microglia, alongside increased proinflammatory cytokines. After four weeks of IL-1β overexpression, they observed a reduction in amyloid pathology, suggesting enhanced microglial clearance of amyloid-beta (Shaftel, 2007; Shaftel, 2008; Matousek, 2012).
Besides the direct implication of IL-1β within the brain, peripheral systemic inflammation can also influence and increase IL-1β levels in the brain, thereby potentially impacting the pathology of AD (Wang, 2023). Various mouse model studies indicate that systemic agents, such as lipopolysaccharide (LPS), can elevate Aβ deposition and cause cognitive deficits (Xie, 2021).
Due to the involvement of IL-1β in AD, it has emerged as a potential biomarker, but studies have shown mixed results regarding its levels in cerebrospinal fluid (CSF) and brain tissue of affected patients (Ng, 2018; Scarabino, 2020). Ultimately, further investigation is necessary to elucidate the intricate relationship between IL-1β, neuroinflammation, and the various pathological hallmarks of Alzheimer’s disease. Understanding the specific mechanisms by which IL-1β influences tau pathology and amyloid clearance could pave the way for the development of targeted therapies against this devastating condition.
Representative Diseases Associated with Inflammasome Activation.
Inflammasome dysregulation is linked to a wide spectrum of diseases, including neurodegenerative, cardiovascular, autoimmune, and metabolic conditions. The figure highlights key examples, though many other diseases also involve inflammasome pathways.
Parkinson's Disease (PD)
PD is a neurodegenerative condition characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra of the basal ganglia, located in the midbrain. This degeneration is associated with the aggregation of α-synuclein within neurons.
The pro-inflammatory cytokine, IL-1β, plays an important role in neuroinflammation and neurodegeneration in PD. Its production is tightly linked to the activation of the NLRP3 inflammasome in microglia. Misfolded α-synuclein activates microglial TLR2/TLR4 signalling, leading to inflammasome assembly, caspase-1 activation, and conversion of pro-IL-1β to its active, secreted form (Koprich, 2008; Pott Godoy, 2008; Leal, 2013; Stojakovic, 2017; Tansey, 2022; Dzamko, 2023). Elevated IL-1β is detected in dopaminergic regions of the brain, CSF, and blood, correlating with disease severity and symptom progression, including cognitive impairment (Dursun, 2015; Liu, 2022; Dzamko, 2023; Qu, 2023). Additionally, other pro-inflammatory cytokines, such as TNF-α, IFNγ, IL-6, IL-2, and IL-10, have also been found to be elevated in plasma (Xu, 2024).
In the context of PD, microglia are the primary producers and secretors of IL-1β:
- Chronic activation perpetuates neuroinflammation via the release of IL-1β, TNF-α, IL-6, reactive oxygen species (ROS), nitric oxide, and TGF-β, creating a toxic milieu for dopaminergic neurons in the substantia nigra and striatum (Leal, 2013; Tansey, 2022; Liu, 2022).
- Robust microglial activation, including transition to phagocytic states, has been observed in PD animal models, promoting a cycle of neuronal damage and further inflammation (Ferrari, 2006; Pott Godoy, 2008).
- Genetic mutations, such as LRRK2 and PINK1, can enhance microglial IL-1β production and exacerbate neuroinflammation (Liu, 2022).
Astrocytes also contribute to PD neuroinflammation:
- Although microglia lead IL-1β production, astrocytes upregulate cytokines, including IL-1β and IL-6, upon inflammatory stimulation and can potentiate microglial responses through NF-κB-dependent pathways.
- Astrocytic activation increases with chronic inflammation, contributing to astrogliosis, scar formation, and modulation of the inflammatory milieu.
- The astrocyte-microglia crosstalk amplifies dopaminergic toxicity (Ferrari, 2006; Leal, 2013).
Collectively, the IL-1β/inflammasome axis, driven predominantly by microglia and supported by astrocytes, propagates chronic neuroinflammation, dopaminergic neuron loss, and PD symptom progression (Leal, 2013; Tansey, 2022; Dzamko, 2023).
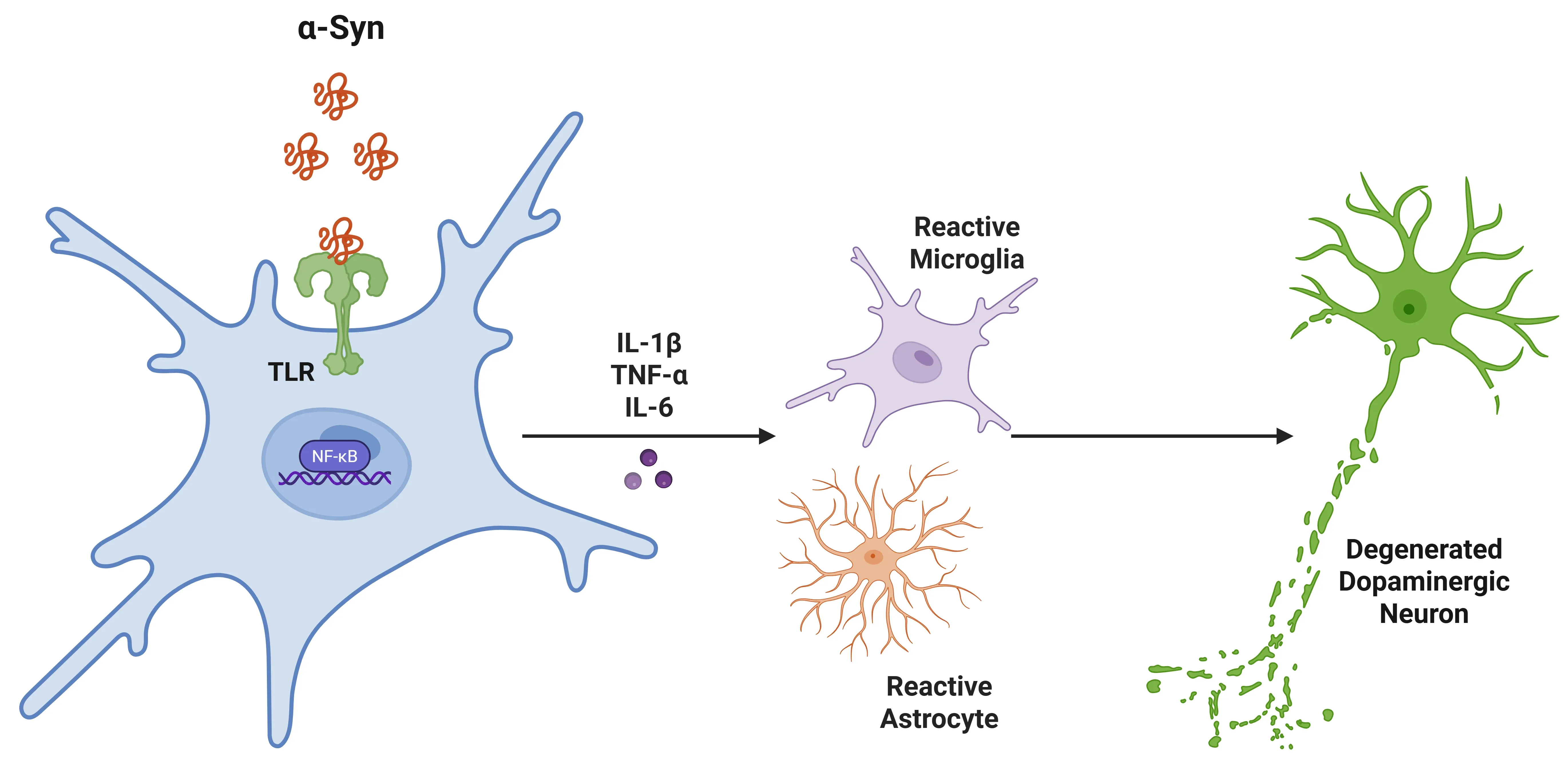
IL-1β in Parkinson's Disease (PD).
Alpha-synuclein binds to microglial toll-like receptors (TLRs), triggering a signalling cascade that activates NFκB. This results in increased pro-inflammatory cytokines, which recruit further microglia and astrocytes, promoting inflammation. Ultimately, this leads to the degeneration of dopaminergic neurons in the substantia nigra.
Amyotrophic Lateral Sclerosis (ALS)
Pro-inflammatory cytokines, such as IL-1β, IL-6, IL-18, and TNF-α, are mediators of neuroinflammation in ALS. Elevated levels of these cytokines have been observed in the CSF and serum of patients as well as in animal models of ALS (Xu, 2024).
TDP-43 and mutant SOD1 (which are linked to familial ALS) can be taken up by microglia, activating a caspase-1-containing inflammasome that processes pro-IL-1β into its active form, IL-1β. This process triggers a cascade of neurotoxic inflammation, thereby accelerating disease progression (Fogal, 2008; Meissner, 2010; van der Meer, 2010; Italiani, 2014; Olesen, 2020; Garofalo, 2022). Astrocytes also contribute to neuroinflammation by adopting reactive phenotypes, modulating inflammatory gene expression, and influencing the inflammatory environment, although their direct involvement in IL-1β production is less significant (Meissner, 2010; Garofalo, 2022).
CSF and serum levels of IL-1β correlate with more aggressive forms of the disease, particularly in specific genetic subtypes such as C9orf72 ALS. In contrast, tissue-specific effects of IL-1β are more pronounced in sporadic ALS (Italiani, 2014; Olesen, 2020). Principal component analyses of CSF in ALS patients indicate that IL-1β is part of a broader inflammatory signature, which includes IL-2, IL-6, IL-13, TNF-α, and others.
There remains ongoing debate about whether neuroinflammation is a primary driver or secondary consequence of motor neuron degeneration due to the variability in IL-1β detectability in human samples compared to animal models, along with IL-1β's dual roles, which can be either neurotoxic or neuroprotective depending on the context and timing (Italiani, 2014; Boraschi, 2023; Femiano, 2024).
Overall, IL-1β and associated inflammatory molecules contribute to neuronal vulnerability through mechanisms involving neurotoxicity, oxidative stress, and synaptic dysfunction, thereby underlining inflammation as a factor in the progression of ALS.
What therapeutic interventions target IL-1β in the treatment of neurodegenerative diseases?
IL-1β plays a critical role in neuroinflammation associated with various neurodegenerative diseases. As a result, IL-1β has been targeted to reduce neuroinflammation, primarily through the use of IL-1 receptor antagonists (IL-1Ra). The main IL-1Ra options include:
- Anakinra: This is a human IL-1Ra that inhibits the activity of both IL-1α and IL-1β.
- Rilonacept: This drug combines the extracellular parts of human IL-1R1 and IL-1R3 with the Fc portion of human IgG1 to inhibit IL-1α and IL-1β long-term.
- Canakinumab: This is a human monoclonal IgG1 antibody that specifically targets IL-1β.
In animal models of AD:
- Chronic administration of an IL-1 receptor blocking antibody in 3xTg-AD mice has been shown to reduce brain inflammation, mitigate cognitive impairment, and diminish tau pathology, while partially lowering certain forms of amyloid-beta. These changes correlated with reduced NF-κB activity and decreased levels of phosphorylated tau and tau kinases (Kitazawa, 2011).
- It is important to note that IL-1β is necessary for the clearance of amyloid-beta, which may partly explain the mixed results obtained in treating AD with IL-1β blockade (Rivera-Escalera, 2019).
In different rat models of PD:
- Peripheral administration of Anakinra and AAV overexpression of IL-1Ra have demonstrated promise in reducing dopamine neurodegeneration and pro-inflammatory cytokines (Dzamko, 2023).
- The injection of lipopolysaccharide into the SN following exposure to 6-hydroxydopamine resulted in increased neurodegeneration and motor symptoms, which were inhibited by IL-1Ra treatment (Koprich, 2008).
In animal models of ALS:
- Preclinical studies in SOD1 transgenic mice showed that Anakinra prolonged survival and improved motor function during the active phase of the disease (van der Meer, 2010; Meissner, 2010).
- Blocking IL-1Ra in mouse models of ALS has also been demonstrated to prolong survival but does not affect disease onset (Maier, 2015).
- While blocking IL-1β reduces neuroinflammation, translating these findings to human ALS is challenging due to issues such as poor CNS drug penetration and the rapid development of treatment-neutralizing antibodies. High doses of Anakinra are necessary to reach the CNS effectively (van der Meer, 2010; Maier, 2015).
Other treatments that reduce IL-1β levels and microglial activation include the administration of dexamethasone (an anti-inflammatory steroid) and MCC950 (an NLRP3 inhibitor) (Pott Godoy, 2008; Liu, 2022).
In summary, targeting IL-1β-driven inflammation holds therapeutic potential. However, the complexity of IL-1β's pleiotropic functions (both toxic and protective) suggests that general anti-IL-1β therapies may have limited success. More recent therapies targeting IL-1β, such as Rilonacept and Canakinumab, are still under investigation and show promise for the future treatment of neurodegenerative diseases.
Our team would be happy to answer any questions about Interleukin-1 Beta (IL-1β) or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Interleukin-1 Beta (IL-1β) and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Inflammasome – A Therapeutic Target for Multiple Diseases
An overview of inflammasomes, including their mechanisms of action, roles in diseases, and targeting for drug development.
What is NLRP3?
An overview of NLRP3 inflammasome activation triggers, disease associations, and therapeutic targeting strategies.
What Is IL-1β (IL-1b)? Function, Signaling, and Biological Role
An overview of IL-1β, including its signaling pathways, involvement in disease mechanisms, and potential therapeutic targets.
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.