Neurodegeneration & Neuroinflammation in the AAV-Synuclein Mouse Model
Biospective’s validated Parkinson’s disease models (α-syn PFF, AAV-A53T, transgenic) are optimal for translational neuroscience drug development. Featuring alpha-synuclein aggregates, neuroinflammation, and neurodegeneration, our PD models enable efficacy, MoA, and target-engagement studies. As a leading global neuroscience CRO, Biospective delivers decision-ready in vivo data supported by advanced imaging, quantitative pathology, behavioral assays, and clinically relevant biomarkers to biotech and pharmaceutical companies.
Biospective specializes in Parkinson's disease animal models, with deep expertise in alpha-synuclein pathology. Recognized as a global preclinical contract research organization, we support biotech and pharmaceutical drug development programs using validated Parkinson’s disease rodent models for efficacy, biodistribution, mechanism-of-action, target engagement, and PK/PD studies across small molecules, antisense oligonucleotides, gene therapy, antibodies, and other biologics.
Biospective’s alpha-synuclein PFF models and AAV-A53T mouse models recapitulate key features of human Parkinson’s disease, including protein aggregation, neuroinflammation, dopaminergic neuron loss, and motor dysfunction. Studies include translational biomarkers such as MRI and PET imaging, fluid biomarkers including neurofilament light chain (NfL), and quantitative IHC. With fully integrated, end-to-end preclinical services, and over a decade of continuous experience executing Parkinson’s disease contract research studies in animal models, Biospective enables translational Parkinson’s disease research from study design through data interpretation.
Why Choose Biospective as Your Parkinson’s Disease CRO?
Biospective is a neuroscience CRO with a focus on Parkinson’s disease animal models, strong scientific expertise, and extensive experience conducting preclinical studies using α-synuclein models.
-
Specialized Parkinson's Disease CRO: Focused exclusively on Parkinson’s and neurodegenerative disease models, not a generalist animal provider.
-
Multiple Validated Parkinson's Disease Models: Transgenic models, viral vector induced, and seeding-based rodent models of Parkinson’s disease are readily available for studies.
-
Alpha-Synuclein Expertise: Deep scientific expertise in α-synuclein biology and pathology, a central misfolded protein in PD.
-
Integrated Services: Fully integrated preclinical services from study design to data interpretation, ensuring seamless execution.
-
Proven Efficacy Data: Industry-standard α-synuclein efficacy datasets and extensive historical controls for robust benchmarking.
-
In-House Transgenic Mouse Colony: Internal colony of A53T α-synuclein transgenic mice (M83 line), enabling rapid study start with well-characterized animals.
-
Accelerated Timelines: Rapid study initiation and efficient workflows to compress timelines without sacrificing quality.
-
Translational Biomarkers: Advanced biomarkers (MRI, PET imaging, CSF/blood assays) that bridge preclinical findings to clinical outcomes.
- Flexible Study Designs: Our scientists work with your team to customize the study design to best fit your goals.
-
Global Support: Experience supporting biotech and pharmaceutical industry clients worldwide, with responsive project management and communication.
Our scientists work as an extension of your internal team, collaborating closely to ensure scientific rigor, reproducibility, and translational relevance at every stage of your Parkinson’s disease research program.
Alpha-Synuclein Mouse Models – Our Core Expertise
Biospective specializes in disease-relevant α-synuclein mouse models for Parkinson's disease drug development.
Alpha-synuclein protein aggregation and propagation are central to Parkinson’s disease pathophysiology. Biospective has built specialized capabilities around α-synuclein–based PD animal models, making this a core differentiator of our CRO services.
These rodent models enable direct evaluation of target engagement and downstream neurodegenerative processes under pathological conditions. Notably, our α-synuclein models recapitulate key features of human Parkinson’s disease – including α-synuclein aggregation, dopaminergic neuron loss, neuroinflammation, motor impairments, and even non-motor symptoms such as sleep disturbances. Our animal model portfolio emphasizes reproducibility, well-defined phenotypes, and the integration of behavioral, imaging, biochemical, molecular, and histopathological endpoints to enable comprehensive in vivo Parkinson's disease efficacy studies and exploration of mechanism-of-action.
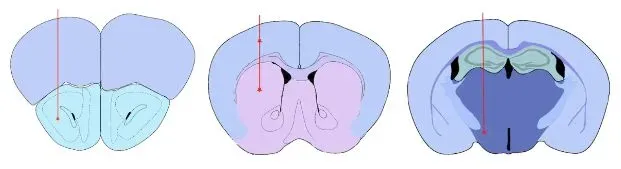
Our validated injection sites: Anterior Olfactory Nucleus (AON). Striatum +/- Overlying Cerebral Cortex, and Medial Forebrain Bundle (MFB).
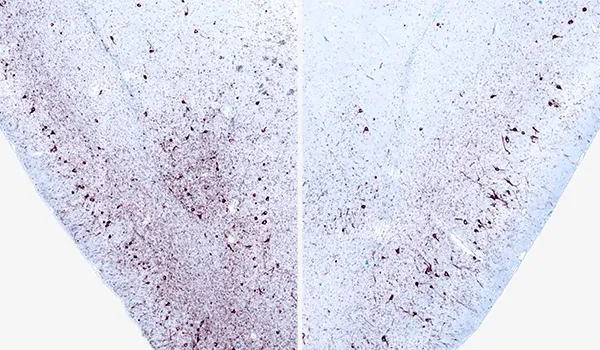
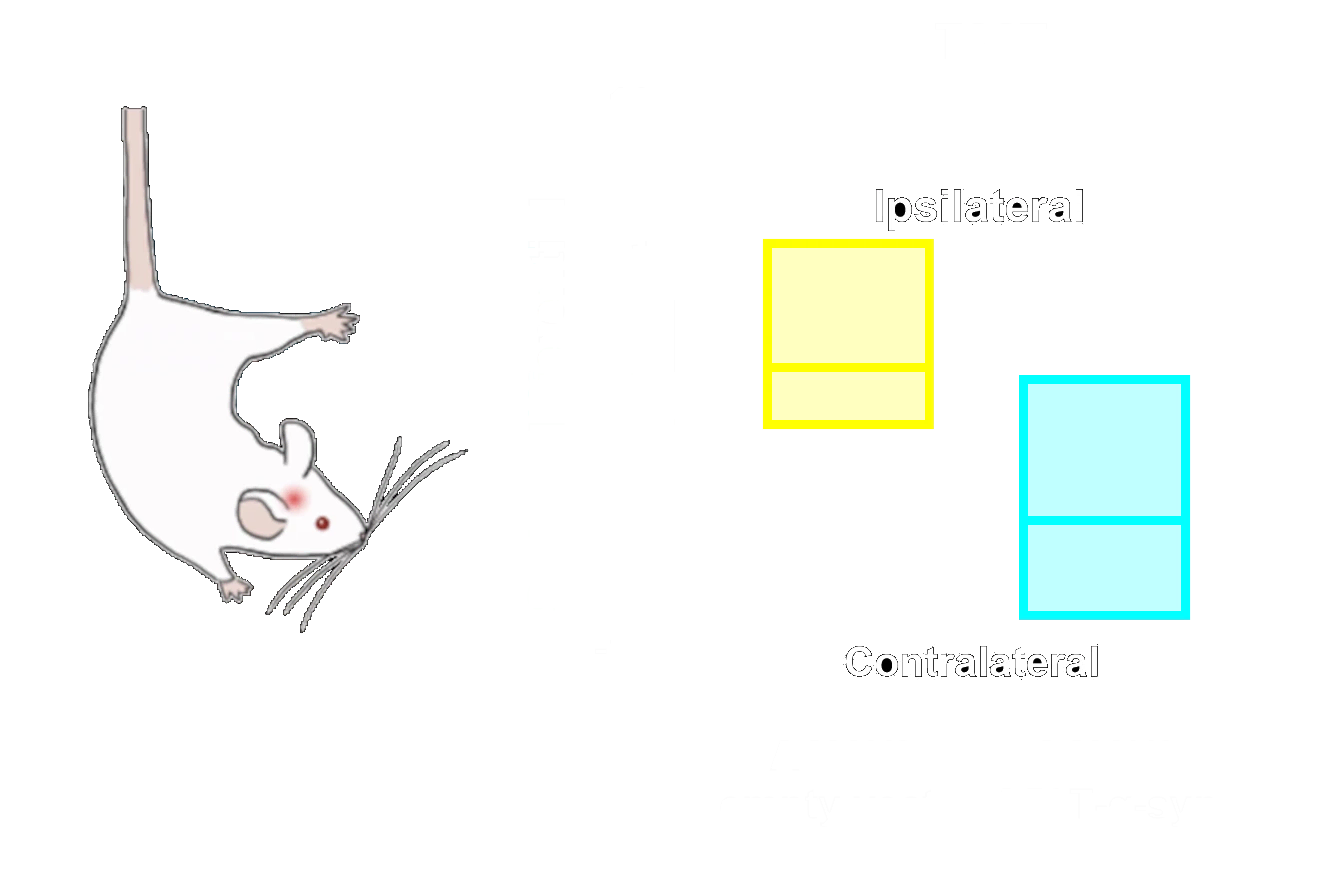
Phosphorylated α-synuclein (pSyn129) IHC of ipsilateral (left) and contralateral (right) piriform cortex 12 weeks after unilateral α-synuclein PFF injection into the AON of an M83+/- mouse.
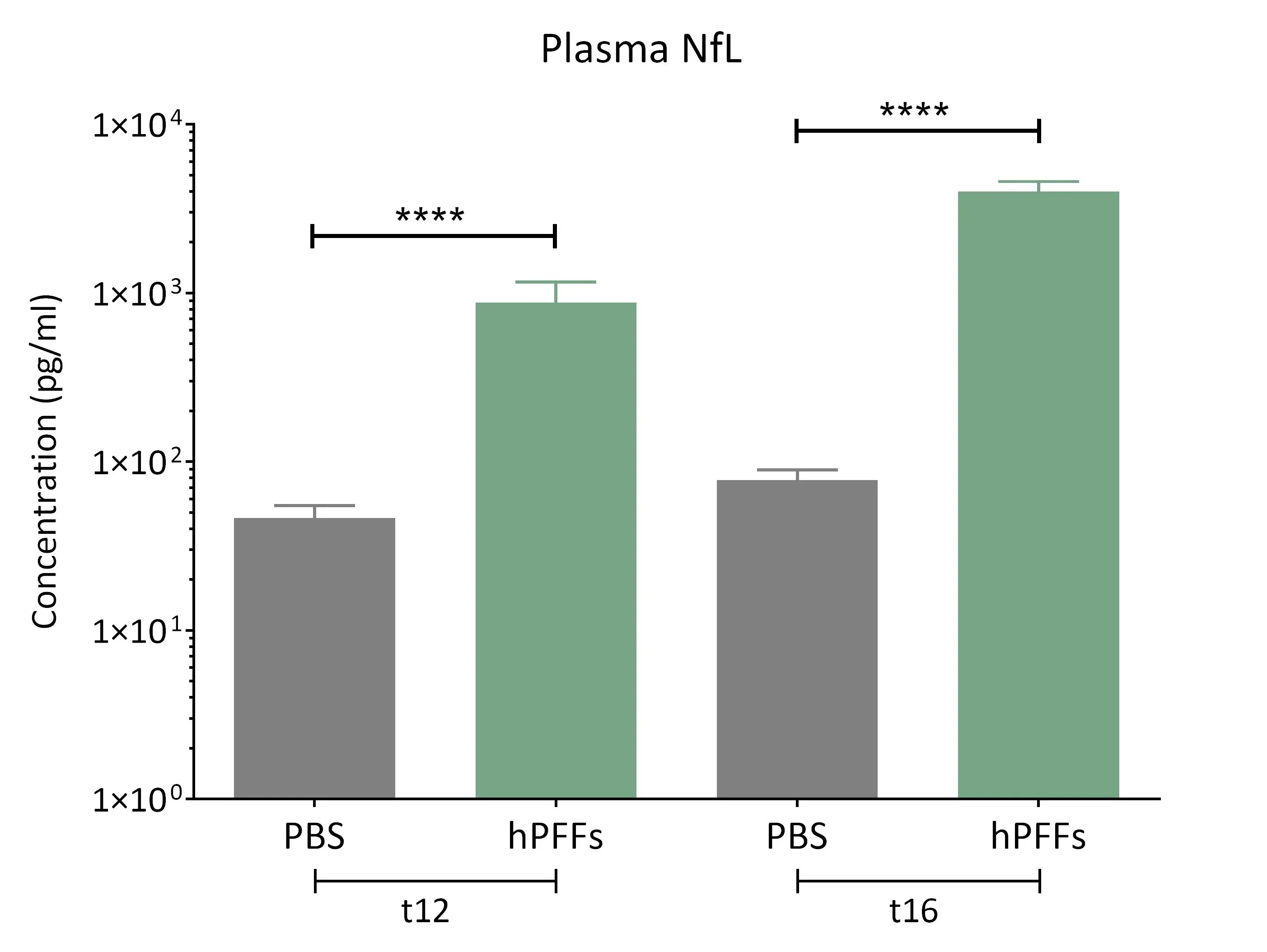
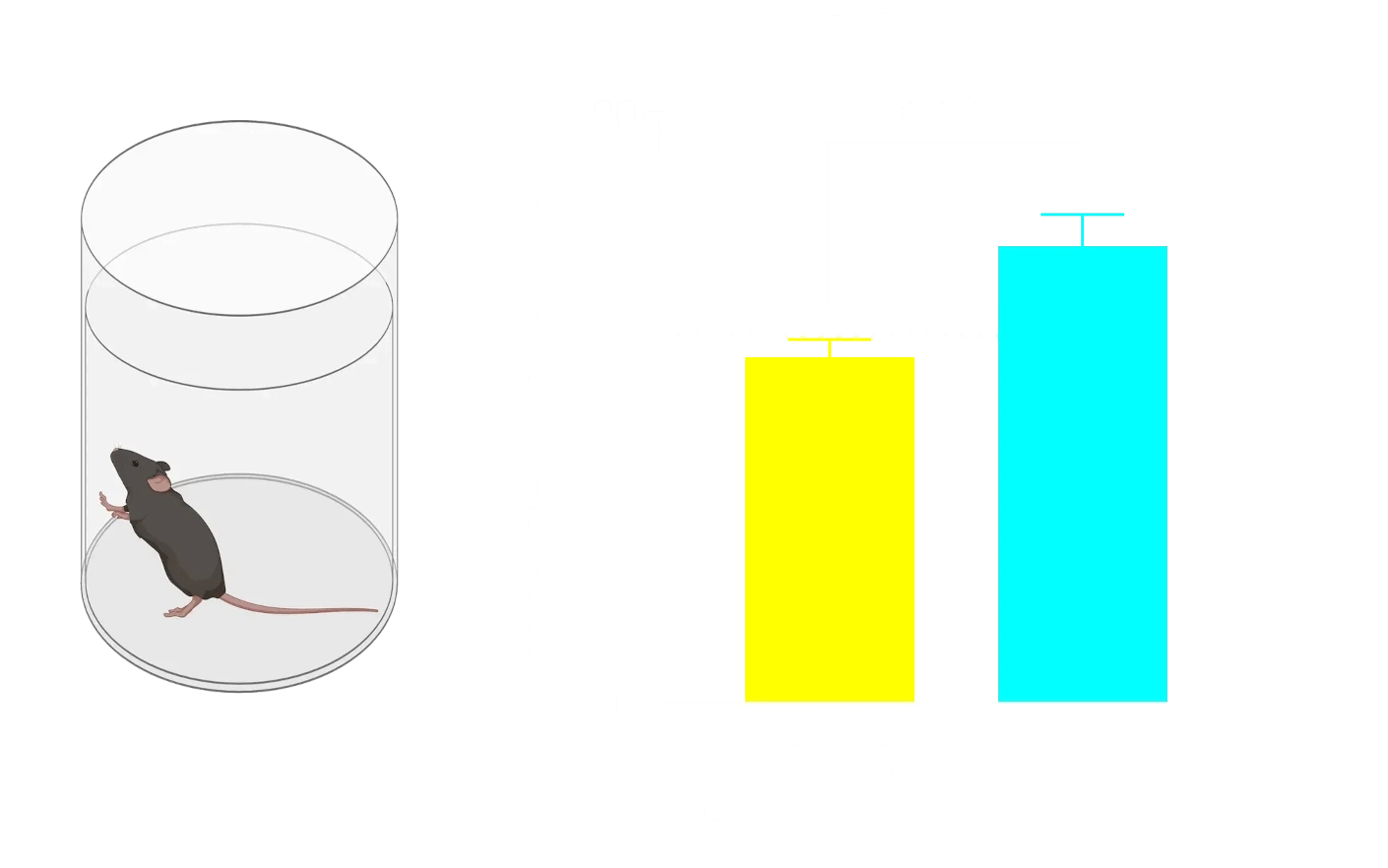
Highly elevated levels of neurofilament light (NfL) are observed in the plasma from the α-synuclein fibril seeding mice.
Alpha-Synuclein Preformed Fibril (PFF) Mouse Model
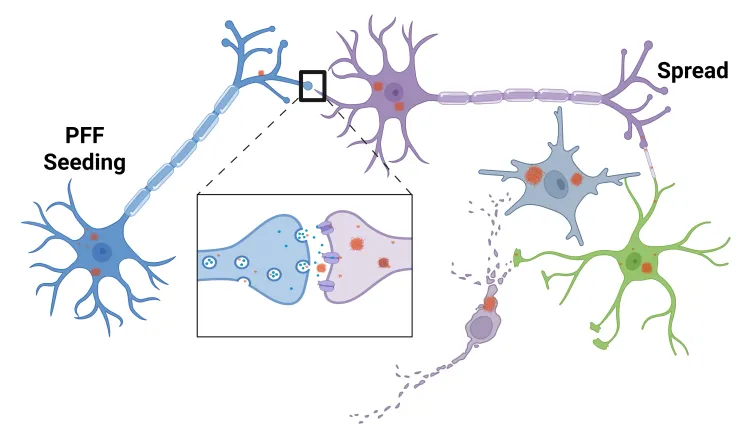
The pathologic spread of misfolded α-synuclein that characterizes human Parkinson’s disease can be recapitulated in rodent brains by stereotaxic injection of α-synuclein preformed fibrils (PFFs). In this PFF seeding and spreading model, exogenous recombinant α-synuclein fibrils seed the aggregation of endogenous α-synuclein and propagate pathology across the brain. This model can be induced in transgenic mice overexpressing human α-syn (e.g. M83 mice with the A53T mutation) as well as in wild-type mice or rats. This model is considered one of the best PD animal models for drug testing given its strong translational relevance.
α-Synuclein PFF Model Induction:
- Injection of recombinant α-synuclein preformed fibrils into the CNS
- Applicable to A53T α-synuclein transgenic mice (M83 line) or wild-type mice
Validated PFF Injection Sites:
- Anterior Olfactory Nucleus (AON) - an early-affected region in PD (Braak stage 1)
- Medial Forebrain Bundle (MFB)
- Striatum (± overlying cortex)
Disease Features Modeled:
- Progressive spreading of α-synuclein pathology in a well-defined spatiotemporal pattern
- Neuroinflammation (microglial and astroglial activation)
- Neurodegeneration (loss of vulnerable neuronal populations)
- Measurable behavioral impairments: motor deficits and non-motor symptoms (e.g. sleep disturbances)
Our α-synuclein PFF-induced PD models are highly reproducible and widely regarded as a gold standard for testing disease-modifying therapeutics. The PFF mouse model is commonly used to evaluate therapies targeting α-synuclein aggregation, propagation, and neurodegeneration in Parkinson’s disease. Biospective has 10+ years of experience executing preclinical studies with α-syn PFF models to evaluate therapeutic biodistribution, target engagement, mechanism of action, and efficacy.
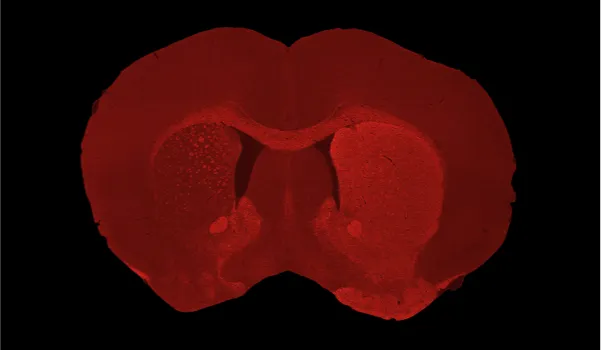
Severe dopaminergic neuron loss and dopaminergic denervation in the ipsilateral (left hemisphere) caudate-putamen following unilateral AAV-hA53Tα-Syn injection into the SNc of a C57BL/6 mouse.
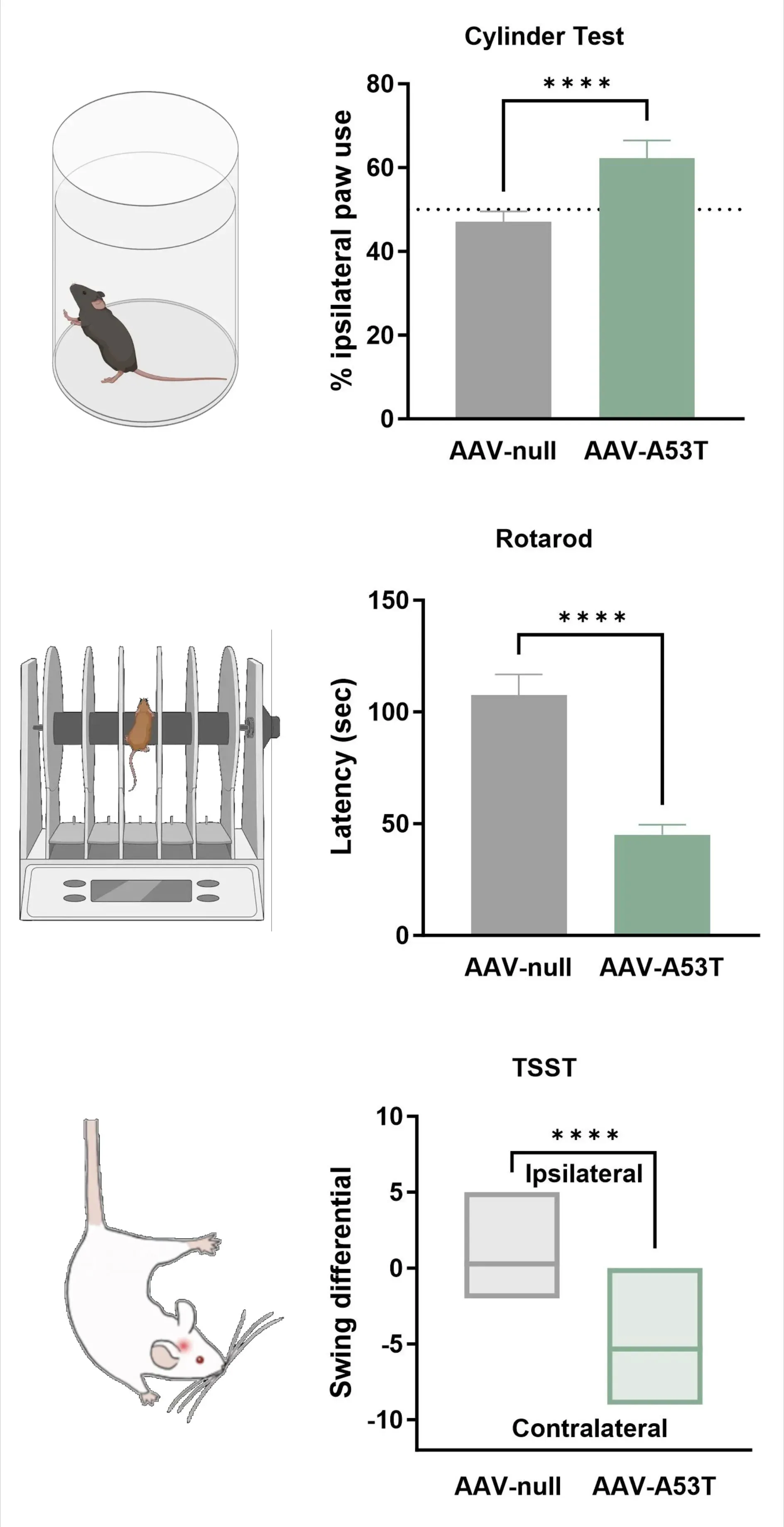
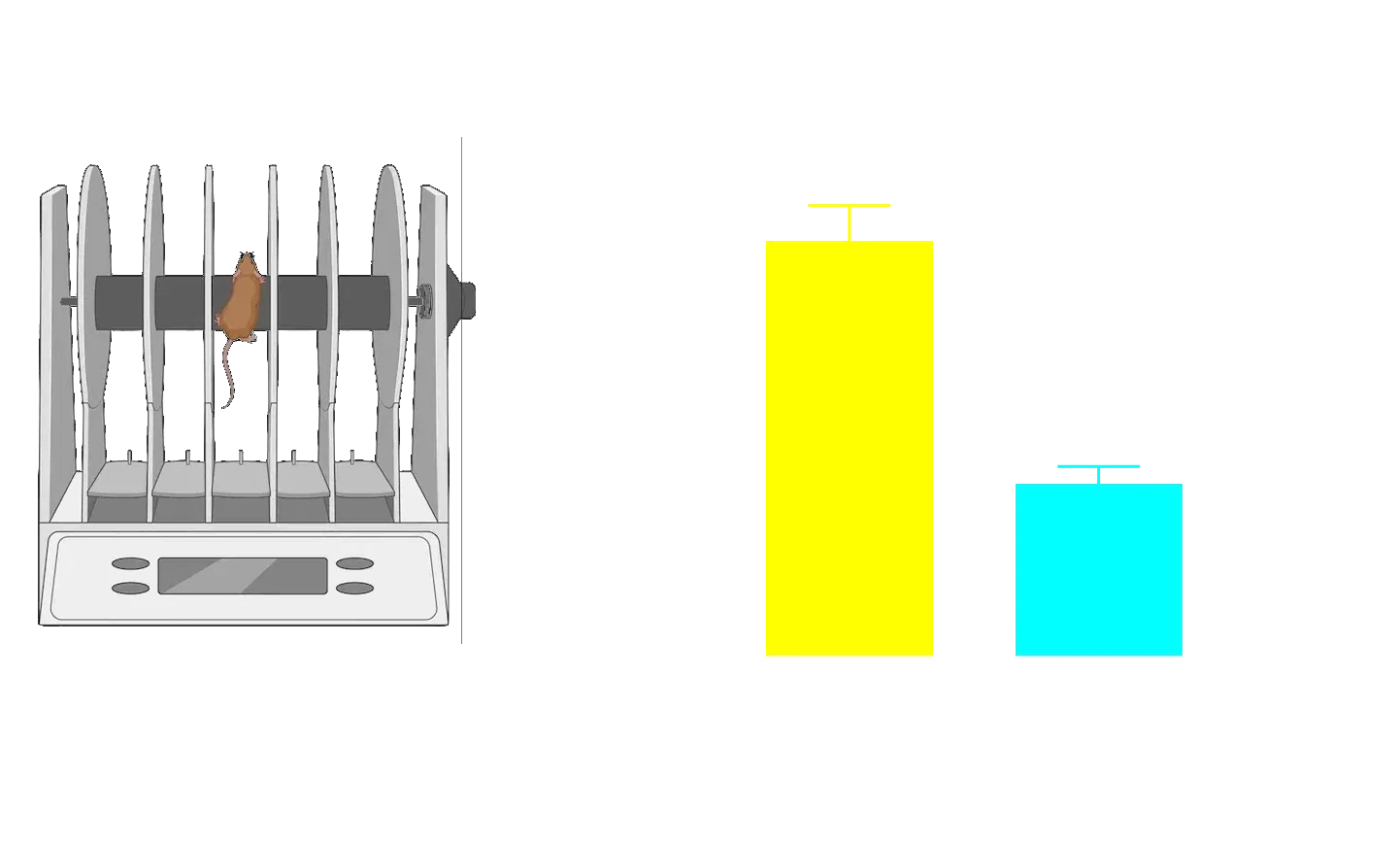
Loss of dopaminergic innervation corresponds with unilateral motor deficits, including increased ipsilateral paw use (Cylinder Test), reduced latency to fall (Rotarod), and increased contralateral swings (Tail Suspension Swing Test).
AAV-A53T Alpha-Synuclein Mouse Model
Generation of α-synuclein pathology in the adult rodent brain can also be achieved via injection of adeno-associated virus (AAV) vectors encoding mutant α-synuclein. In this PD mouse model, adult wild-type (C57BL/6) mice (or suitable transgenic backgrounds) receive a unilateral stereotaxic injection of an AAV vector overexpressing human α-synuclein with the A53T mutation directly into the substantia nigra pars compacta (SNc). Our skilled surgical team uses high-precision digital stereotaxic guidance with automated microinjectors for accurate viral delivery to the target region. This model replicates dopaminergic neuron loss and other hallmark phenotypes of Parkinson's disease, measurable via translational biomarkers, making it among the best PD animal models for drug evaluation.
AAV-A53T α-Syn Model Induction:
- Unilateral stereotaxic injection of AAV expressing human A53T α-synuclein into the SNc
Validated Injection Site:
- Substantia Nigra pars compacta (SNc) – to induce nigrostriatal degeneration
Disease Features Modeled:
- Dopaminergic neuron loss in the SNc and striatum
- Phosphorylated α-synuclein aggregates in the SNc and striatum
- Neuroinflammation (local microglial and astroglial activation)
- Neurodegeneration (progressive loss of nigral neurons)
- Unilateral motor deficits observable via behavioral tests, including the Cylinder Test, Tail Suspension Swing Test (TSST), Hindlimb Clasping Test, Rotarod Test.
In addition to standard endpoints, Biospective can perform non-invasive imaging studies on AAV α-synuclein mice – such as MRI volumetric analysis and PET imaging (e.g. [18F]FDG PET for glucose metabolism, [18F]DOPA PET for dopaminergic function) – to generate clinically translational imaging biomarkers (e.g. regional brain atrophy, cerebral hypometabolism, striatal dopamine terminal loss). This AAV-A53T model is also excellent for high-throughput studies (including screening studies) of disease-modifying therapeutics, thanks to its rapid start-up (no breeding needed), use of wild-type animals, cost-effective induction, and suitability for large cohort studies.
Translational Pathology and Biomarkers in Parkinson’s Disease Models
Biospective has established a broad range of clinically-relevant disease markers to facilitate translation to clinical studies.
As a Preclinical Neuroscience CRO, we design our PD models with translational relevance to mirror key aspects of the human disease. A major differentiator of Biospective is our focus on translational biomarkers that align preclinical findings with clinical outcomes – including advanced neuroimaging and fluid biomarkers. We incorporate:
-
Alpha-synuclein–related biomarkers (pathology and spread)
-
Neuroinflammation markers (microglial/astrocyte activation)
-
Neurodegeneration endpoints (neuron loss, atrophy)
-
Mechanism-of-action confirmation (target/pathway engagement)
Our modeling and biomarker strategies ensure that preclinical successes meaningfully predict clinical potential, de-risking the transition from animal studies to human trials.
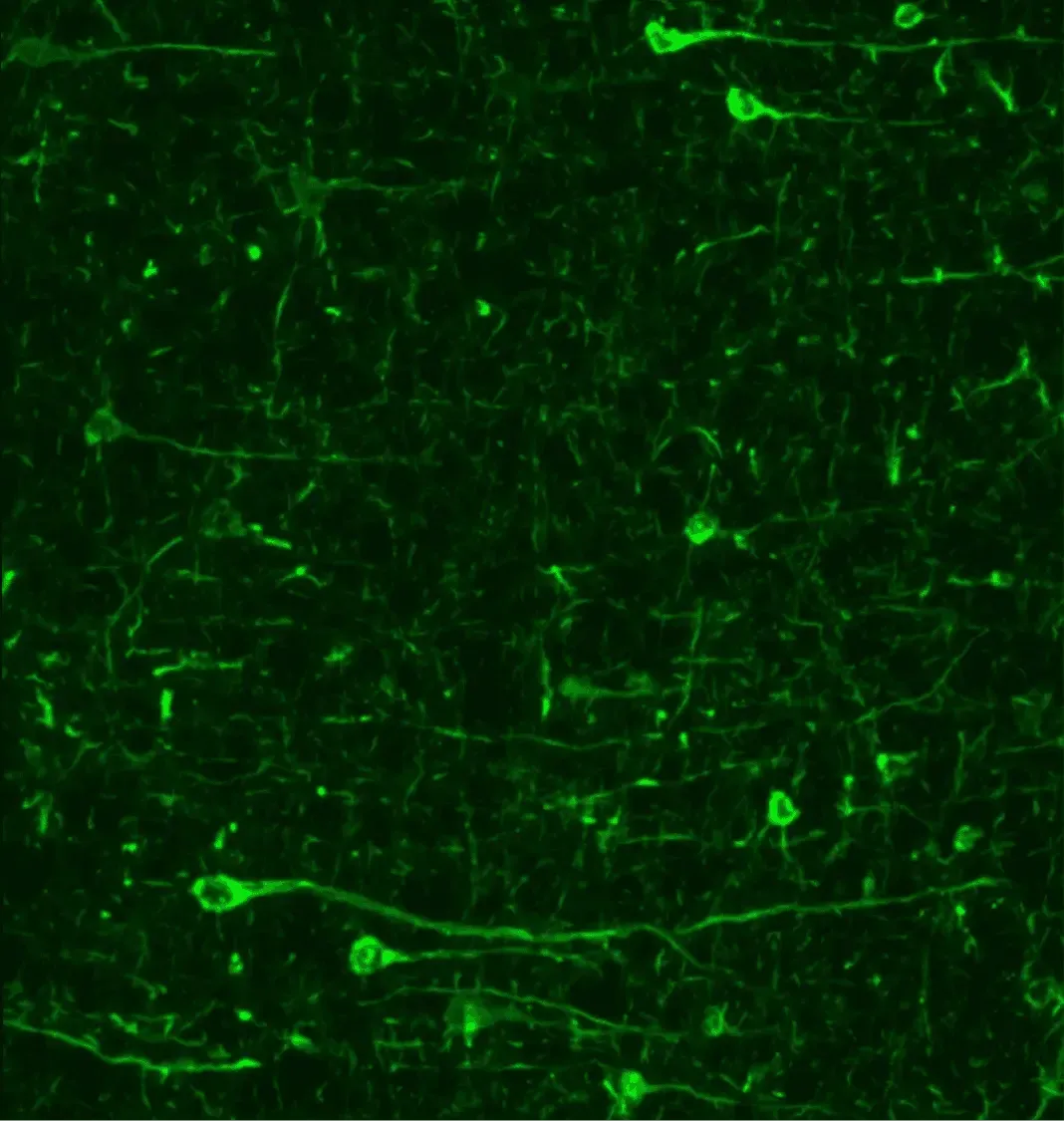
Immunofluorescence of phosphorylated synuclein (pSyn129) in Parkinson's disease animal models reveals pronounced accumulation in neuronal soma and processes.
Alpha-Synuclein Aggregates
Misfolded α-synuclein aggregates are a pathological hallmark of Parkinson’s disease. In patients, Lewy bodies and Lewy neurites (α-syn–rich inclusion bodies) are observed in the dopaminergic neurons of the SNc and in other brain regions, following a characteristic spatiotemporal progression (Braak, 2003). In our PFF- and AAV-induced PD models, we likewise observe robust α-synuclein pathology – including high levels of phosphorylated α-syn (pSyn129) accumulation in neuronal cell bodies and processes, and, in the PFF models, extensive seeded spread of α-syn aggregates throughout connected brain regions.
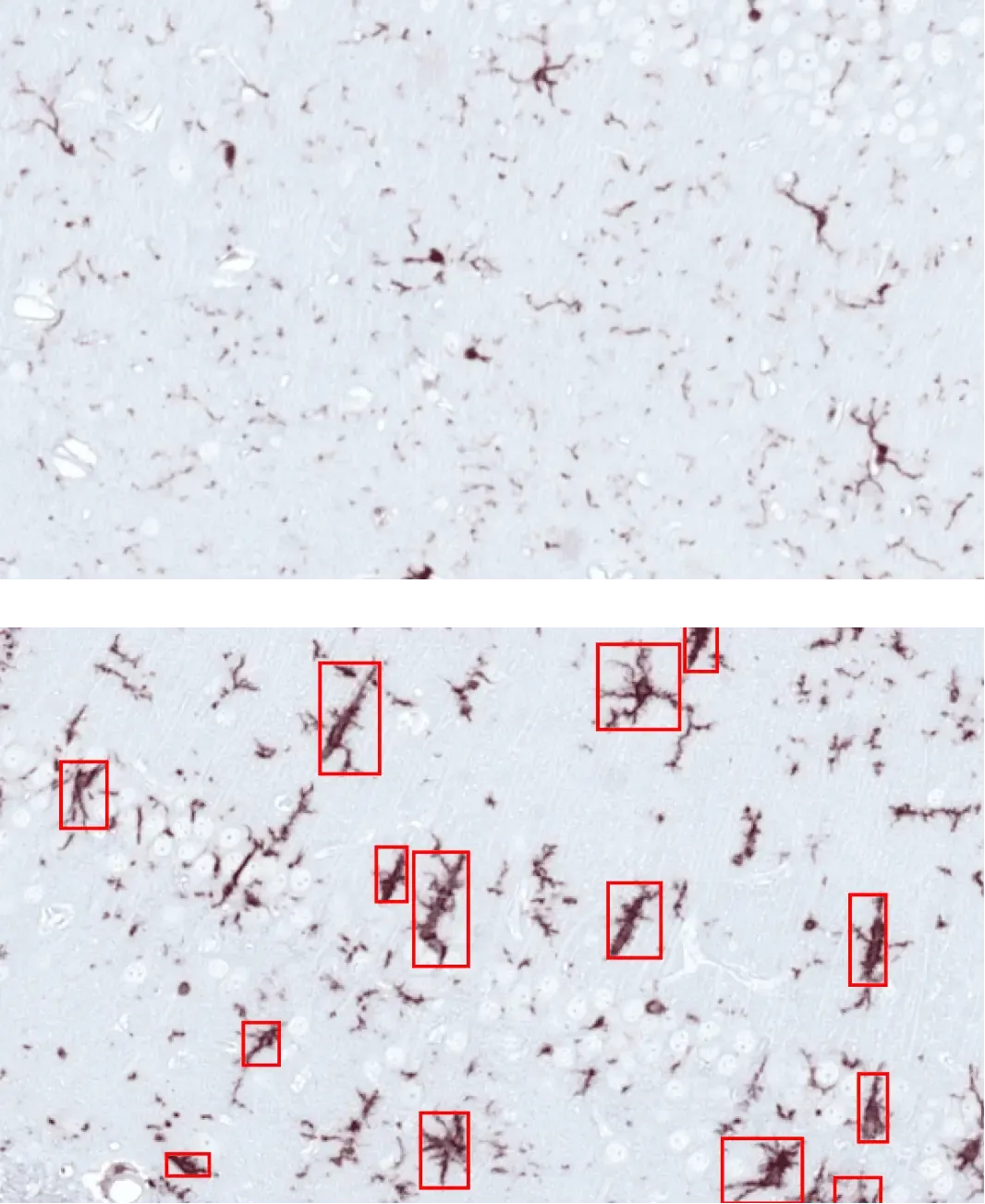
Activated microglia (red boxes) in the hippocampus of mice injected into the AON with PBS (top) or α-synuclein PFFs (bottom).
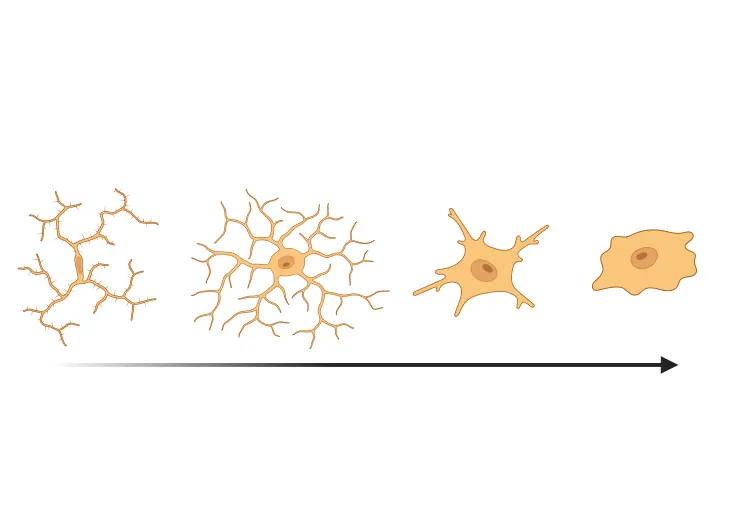
Activated Microglia & Reactive Astrocytes
Neuroinflammation is a key pathologic feature of Parkinson’s disease, with chronic activation of microglia and astrocytes contributing to neurodegeneration (Kam, 2020; Chen, 2023). In our AAV and PFF PD models, we see strong neuroinflammatory responses with distinct spatiotemporal patterns. We apply cutting-edge image analysis – including proprietary computer vision and deep learning algorithms – to detect and quantify changes in microglial and astrocytic morphology in brain tissue. (See our Initiative: Microglia, Astrocytes, and Neurodegenerative Diseases and our Innovation: Microglial Activation in an α-Synuclein Mouse Model of Parkinson's Disease for more details.) These approaches allow sensitive tracking of neuroinflammation in relation to α-syn pathology.
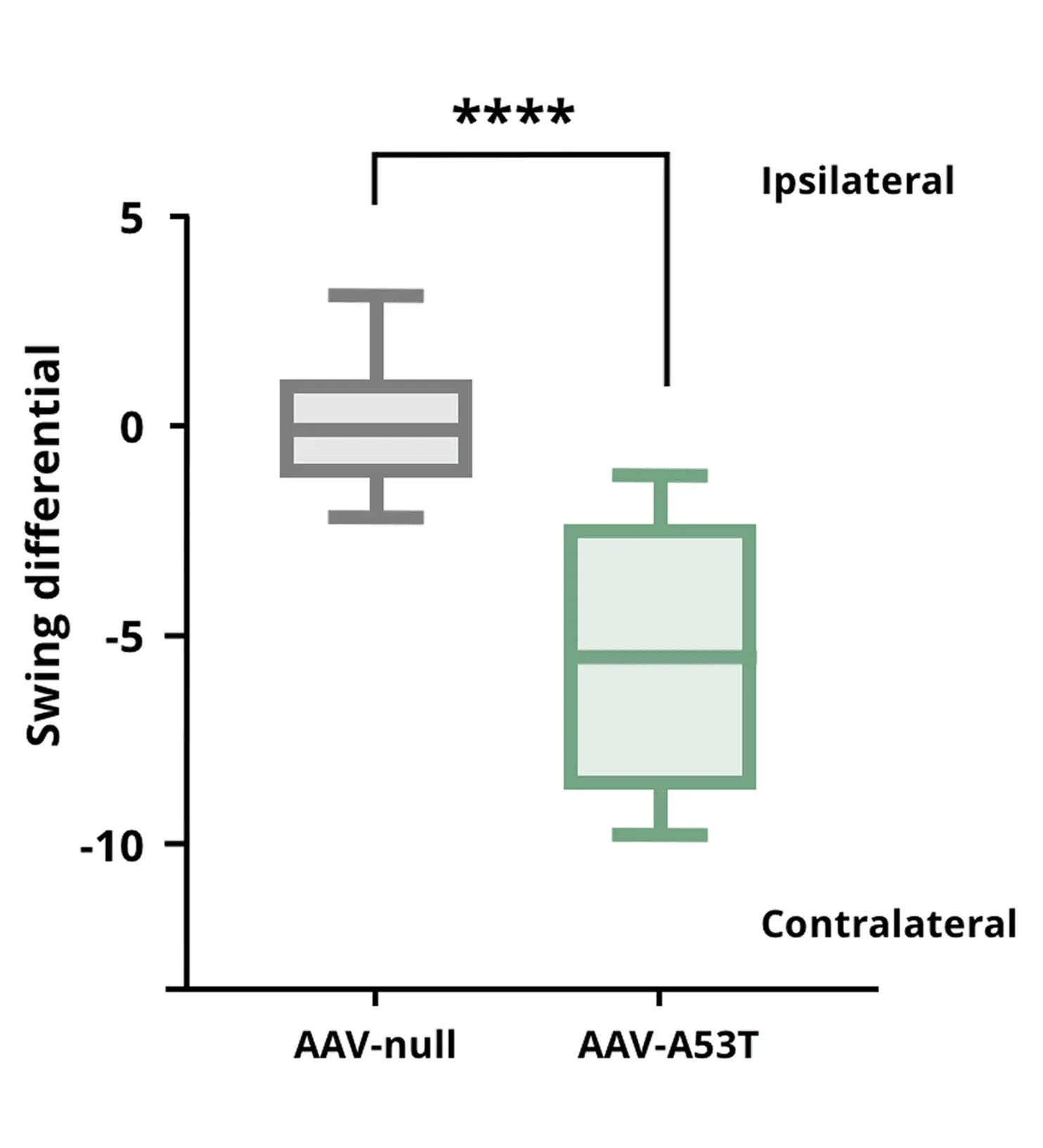
Tail Suspension Swing Test showing that AAV-A53T α-syn mice exhibit increased contralateral swings due to a unilateral dopaminergic deficit, compared to AAV-null control mice.
**** p<0.0001.
Dopaminergic Neuron Loss & Motor Deficits
Extrapyramidal motor symptoms in Parkinson’s disease are primarily driven by the degeneration of dopaminergic neurons in the substantia nigra pars compacta and the resultant loss of dopaminergic projections to the striatum. In our PD models, we induce α-synuclein pathology targeting the SNc via either AAV-mediated gene delivery or PFF injection . Both interventions produce significant dopaminergic neuron loss in the SNc and striatal dopamine terminal loss, leading to motor impairments that we quantify through behavioral assays (e.g. rotarod, tail suspension swing, cylinder, and hindlimb clasping tests). (See our Resource: Preformed Fibrils – A Guide to Cell and Animal Models for additional background on these approaches.) These motor phenotypes in the models parallel the movement disorders seen in human PD, enabling evaluation of therapeutic effects on motor function.
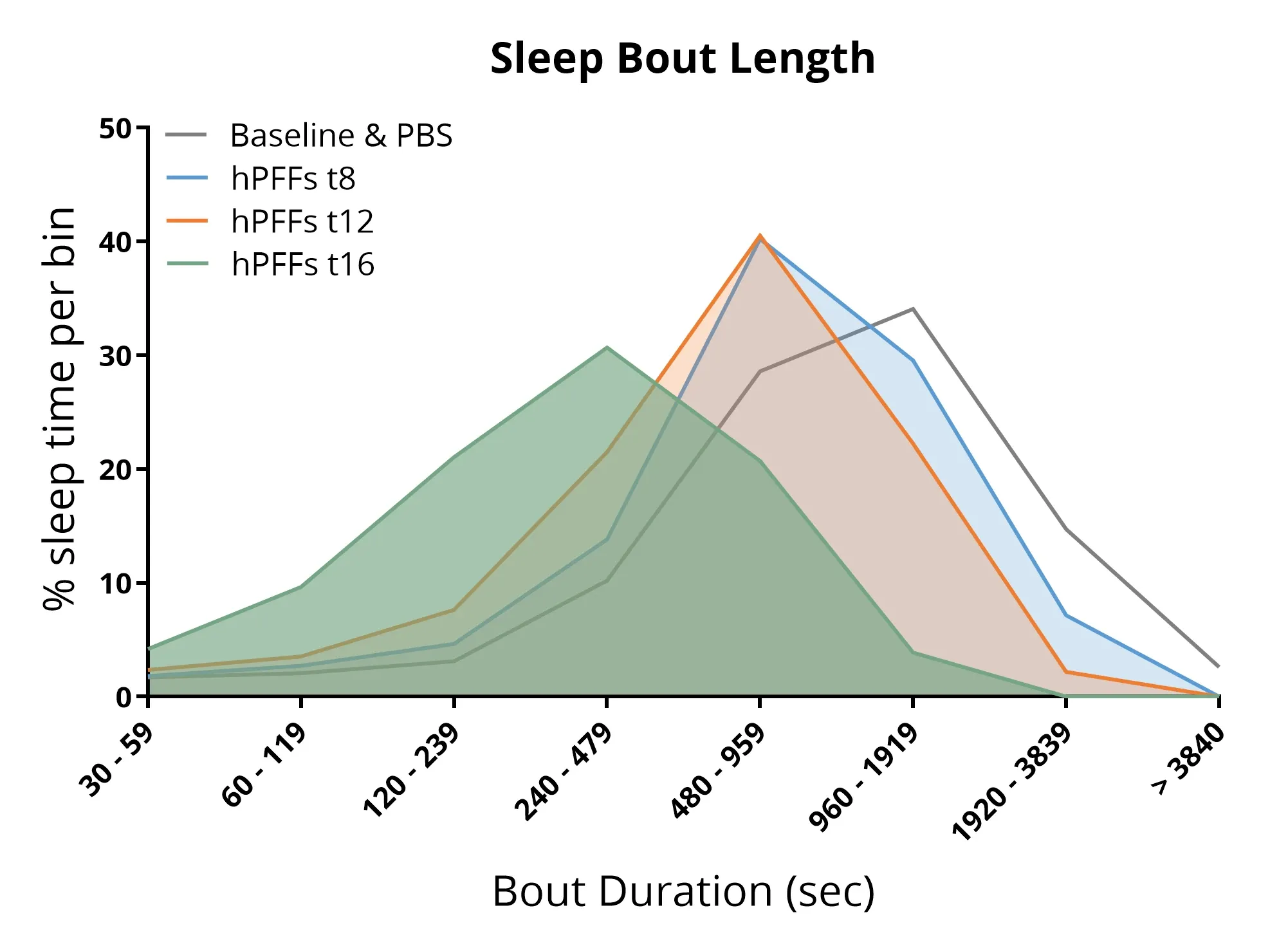
Sleep analysis showing that mice injected with α-syn PFFs into the AON exhibit disrupted sleep architecture, including shorter sleep bout lengths and reduced overall sleep duration.
Sleep Alterations
Sleep disturbances are among the most prevalent non-motor symptoms of Parkinson’s disease, affecting up to ~85% of patients (Stefani, 2020; Asadpoordezaki, 2025). Using a non-invasive sleep monitoring system, we have shown that PFF-seeded α-syn pathology can disrupt sleep-wake architecture in transgenic mice. For example, injecting α-syn PFFs into the AON of A53T (M83) mice leads to altered sleep architecture, including changes in total sleep time and significantly shorter sleep bout lengths (more fragmented sleep). Such findings mirror the insomnia and REM sleep behavior disorders observed in PD patients. (Sleep phenotypes have not yet been evaluated in our AAV model.)
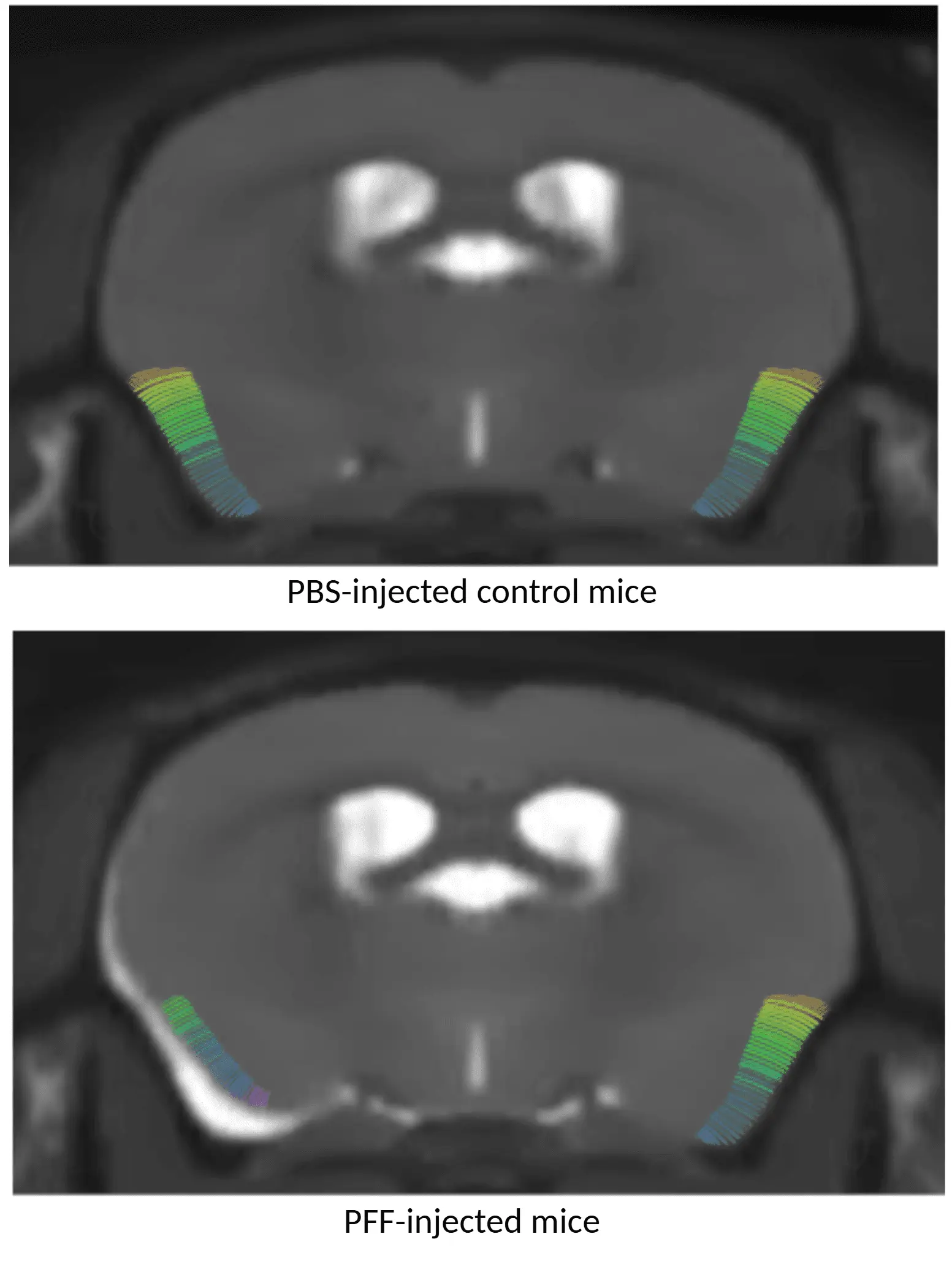
MRI cortical thickness maps showing PBS-injected mice (top) and AON α-syn PFF-injected mice (bottom), with PFF-treated animals displaying cortical thinning.
Regional Brain Atrophy
Neuroimaging biomarkers are widely used in clinical trials of Parkinson’s disease to assess neurodegeneration. Magnetic resonance imaging (MRI) can detect regional brain atrophy and cortical thinning, which are sensitive indicators of neuron loss in PD (Tremblay, 2021; Abdelgawad, 2023). We apply high-resolution MRI with fully automated volumetric analysis to our PD models. Using these techniques, we have demonstrated reproducible brain atrophy in both AAV and PFF mouse models of PD, including significant cortical thinning in PFF-injected mice relative to controls. (See our Innovation: Brain Atrophy Analysis in Mouse Models of Neurodegeneration to explore this capability.) These MRI endpoints provide quantitative, translational biomarkers that can be directly compared to human PD imaging data.
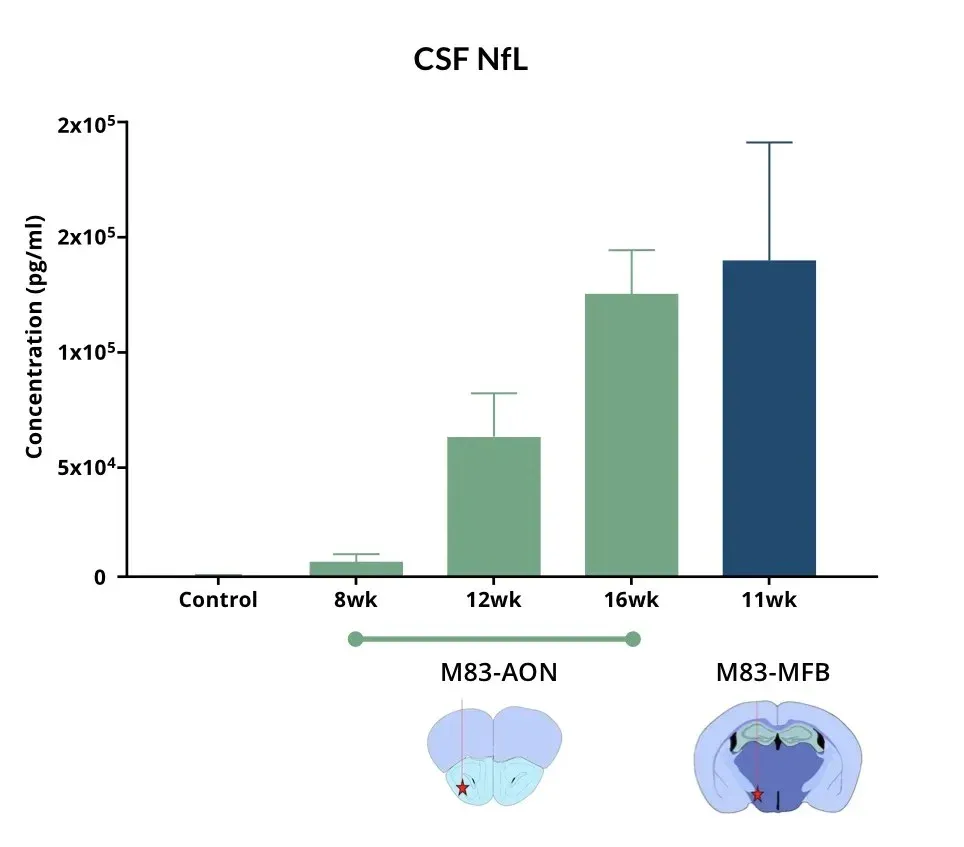
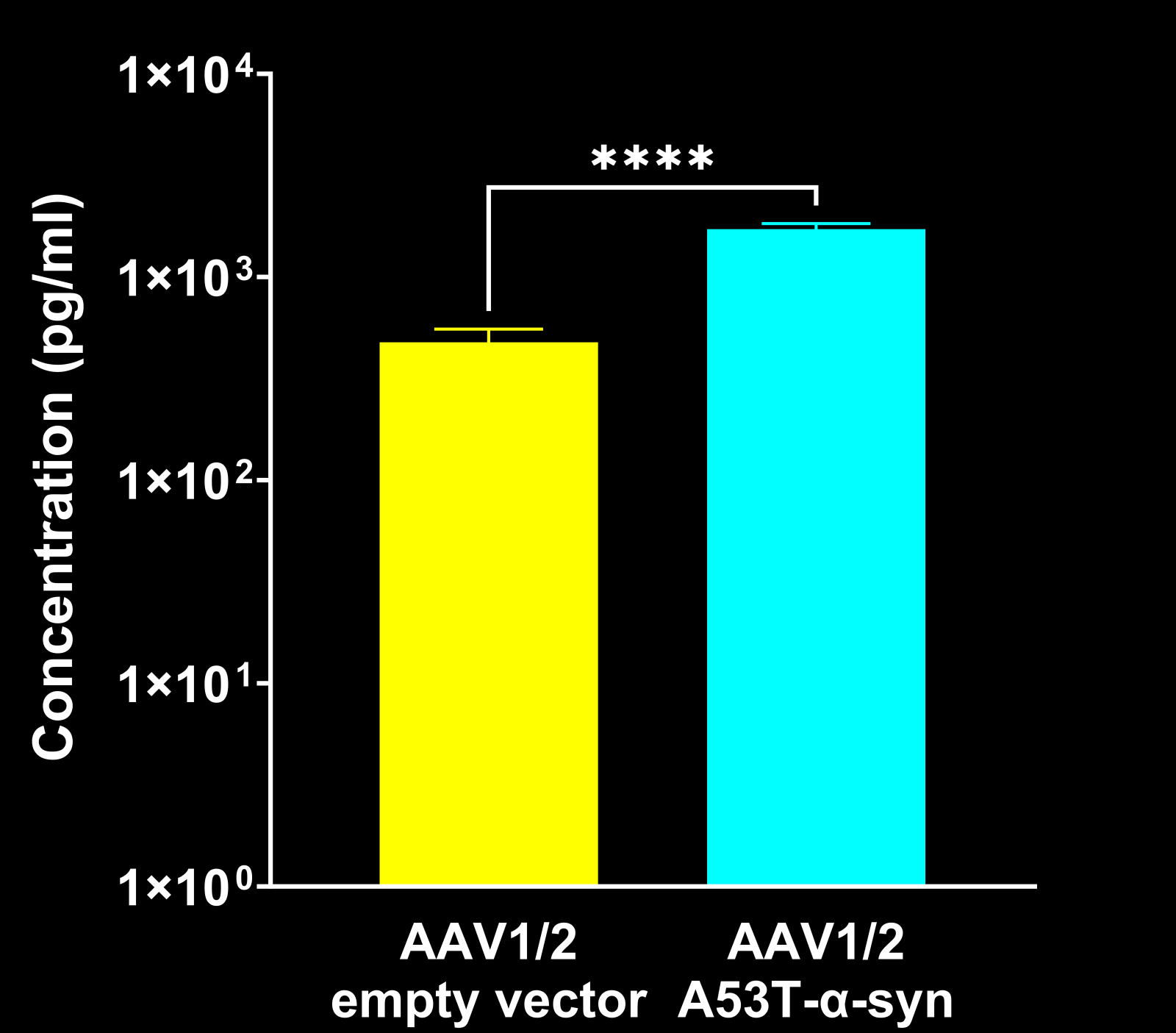
α-Syn PFF-injected mice in the AON and MFB show elevated CSF NF-L levels compared to control mice.
Elevated Neurofilament Light (NfL) in CSF & Plasma
Neurofilament light chain (NfL) is a well-established fluid biomarker of neurodegeneration. NfL levels are elevated in the CSF and plasma of Parkinson’s disease patients, and NfL is frequently measured in PD clinical trials (Bäckström, 2020; Urso, 2023; Pedersen, 2024). Elevated NfL has also been reported in several preclinical PD models. Consistent with clinical findings, our Parkinson’s mouse models exhibit significant increases in NfL in both plasma and CSF. In particular, PFF-injected transgenic mice show marked NfL elevation in biofluids (plasma, CSF) after α-syn fibril injection into regions like the AON or MFB. (See our Resource: Neurofilament Light Chain in Parkinson’s Disease Models for additional data.) This biomarker provides a quantifiable readout of neurodegeneration in our studies, strengthening the clinical relevance of the model outcomes.
Pathologic and Phenotypic Profiles of PFF versus AAV α-Synuclein Parkinson's Disease Models
A comparison of how each animal model of Parkinson's disease reproduces hallmark features of human disease, including their characteristics and use cases.
Pathology
Key PD-associated pathological features (α-syn aggregates, neuroinflammation, dopaminergic neuron loss) in each mouse model.
| Feature/Domain | PFF Models | AAV Models |
|
✔️ |
✔️ | |
|
✔️ |
✔️ | |
|
✔️ |
✔️ |
Functional Features
Behavioral and physiological impairments linked to the pathology.
|
Feature/Domain |
PFF Models |
AAV Models |
|
✔️ |
✔️ | |
|
✔️ |
N/A – Not yet evaluated in AAV model |
Translational Biomarkers
Non-invasive imaging and fluid biomarkers reflecting neurodegeneration.
|
Feature/Domain |
PFF Models |
AAV Models |
|
✔️ |
✔️ | |
|
✔️ |
✔️ |
Model Comparison Summary
Both the PFF and AAV α-synuclein mouse models exhibit robust α-synuclein pathology and significant neuroinflammation, closely mirroring the core features of human Parkinson’s disease. The PFF model uniquely recapitulates the prion-like spread of α-synuclein aggregates through the brain (including involvement of non-dopaminergic regions), whereas the AAV model produces more pronounced, regionally selective dopaminergic neuron loss in the SNc along with earlier-onset, severe motor deficits. Sleep disturbances have been demonstrated in the PFF model (due to its longer timeline and limbic involvement) but have not yet been assessed in the AAV model. Notably, both models support the use of MRI-based brain atrophy measures and elevated NfL in biofluids as translational biomarkers of neurodegeneration, facilitating direct comparison to clinical trial endpoints. Together, the PFF and AAV models provide complementary platforms that allow a comprehensive evaluation of Parkinson’s disease mechanisms and therapeutic interventions across diverse experimental needs.
Illustrative Example of Our Alpha-Synuclein Mouse Models
An Interactive Data Presentation of some of the key motor, imaging, and pathological findings from Biospective’s AAV-A53T α-synuclein mouse model of Parkinson’s disease.
In this example study, 12-week-old C57BL/6 mice were injected unilaterally in the left SNc with either AAV-hA53T-α-synuclein or a control AAV-null vector (2 µL, infused at 0.4 µL/min). A digital stereotaxic system with automated microinjection was used to ensure precise targeting of the SNc (see the coronal atlas view of the injection site).
Multiplex immunofluorescence (mIF) imaging was performed on brain sections, staining for phospho-α-syn (pSyn129), tyrosine hydroxylase (TH), GFAP (astrocytes), Iba1 (microglia), and nuclei (DAPI). Whole-slide high-resolution scans were analyzed using Biospective’s quantitative image analysis software. The results show a substantial loss of TH-positive dopaminergic neurons in the injected (ipsilateral) SNc compared to the contralateral hemisphere, accompanied by dense pSyn129-positive α-syn aggregates and robust glial activation in the affected regions. Quantitative analysis confirmed a highly significant reduction of TH immunoreactivity in the ipsilateral SNc, reflecting the targeted neurodegeneration.
How to use Our Interactive Viewer
Navigate through the “Image Story” via the left-hand panel or the on-screen arrows. You can pan around high-resolution microscopy images with your mouse, and zoom in/out using the scroll wheel or the +/- controls. The Control Panel (top-right) allows toggling of image channels and segmentation overlays. For the best experience, we recommend switching to full-screen mode. This Interactive Presentation enables you to explore the model’s neuropathology and associated functional deficits in detail, as if looking directly down the microscope.
Characterization of our AAV-A53T-Synuclein mouse model, including in vivo data and high-resolution images of entire Multiplex Immunofluorescence tissue sections.
Click to copy link
How are Parkinson's Disease Mouse Models Used in Drug Development?
We work closely with our biotech and pharmaceutical sponsors to:
-
Evaluate therapeutic efficacy and dose-response in Parkinson’s models
-
Assess target engagement and disease-modifying effects
-
Support translational biomarker strategies, including imaging and fluid biomarkers for clinical readiness
Our Parkinson’s disease mouse models are optimized for in vivo testing of multiple therapeutic modalities, including both traditional and advanced approaches:
Small Molecules
-
Brain penetration and PK/PD profile
-
Behavioral efficacy on motor and non-motor symptoms
-
Reduction of pathological hallmarks (α-syn aggregates, neuron loss)
RNA-Targeted Therapies
- Target knockdown verification (e.g. mRNA or protein level reduction)
-
CNS biodistribution of ASOs/siRNA
-
Translational biomarker readouts to confirm pathway engagement
Gene Therapy & Viral Vectors
-
Transgene expression levels in target regions
-
Regional biodistribution of viral vectors (e.g. AAV spread)
-
Functional rescue or disease modification outcomes (behavioral and pathological improvements)
Antibodies & Biologics
-
CNS exposure and penetration of biologics (e.g. BBB engagement)
-
α-Synuclein aggregation clearance or reduction
-
Mechanism-of-action validation (target binding, downstream signaling changes)
End-to-End Parkinson’s Disease Preclinical CRO Services
Biospective offers fully integrated preclinical Parkinson’s disease contract research services.
-
Study design & model selection – expert guidance on choosing the right PD model and designing robust studies
-
In vivo efficacy studies – execution of treatment studies with comprehensive monitoring of outcomes
-
Biodistribution & PK/PD – analysis of drug distribution and pharmacokinetics/pharmacodynamics in CNS and periphery
-
Target engagement assays – confirmation that the therapeutic hits its molecular target (e.g. α-syn reduction, pathway modulation)
-
Behavioral analysis – motor and non-motor behavioral testing (rotarod, gait, grip strength, sleep monitoring, etc.)
-
In vivo multi-modality imaging – MRI, PET, SPECT, fluorescence, and bioluminescence imaging to track disease and treatment effects
-
Immunoassays – biomarker quantification in CSF, blood, and tissue (e.g. NfL, cytokines, chemokines)
-
Immunohistochemistry (IHC) & multiplex immunofluorescence (mIF) – post-mortem tissue staining & quantitative image analysis to assess pathology and therapeutic impact
-
Data analysis & reporting – rigorous quantitative analysis, statistics, and comprehensive reporting by our scientists
This end-to-end approach minimizes handoffs, accelerates timelines, and reduces risk for our sponsors by keeping all aspects of the study under one expert team.
Parkinson's Disease Animal Models Summary: How do PFF and AAV α-Synuclein Models Compare?
Together, the PFF and AAV α-synuclein rodent models provide complementary platforms for investigating Parkinson’s disease mechanisms and evaluating novel therapeutics. Both models faithfully reproduce core features of PD – including robust α-synuclein aggregation and measurable neuroinflammation – while each has unique strengths in terms of phenotype expression and timing. The PFF model offers a powerful system for studying the prion-like seeding and spreading of α-synuclein pathology across the brain over time, including limbic and cortical regions. In contrast, the AAV model produces pronounced, selective dopaminergic neuron loss in the SNc and rapid-onset motor deficits, more directly modeling the nigrostriatal degeneration responsible for classic PD motor symptoms. Sleep and other non-motor phenotypes have been demonstrated in the PFF model (over longer durations), whereas these endpoints remain to be characterized in the faster AAV model. Importantly, both models support translational endpoints – such as MRI-detected brain atrophy and elevated NfL in biofluids – that bridge preclinical findings to clinical measures of neurodegeneration. By leveraging both models, researchers can comprehensively evaluate Parkinson’s disease pathways and therapeutic effects, from molecular mechanisms to functional outcomes.
Learn more about our in-depth characterization of these Parkinson’s disease mouse models, our validated outcome measures, and the full scope of our Parkinson's disease CRO services.
Related Content
Explore up-to-date insights on Parkinson’s disease and best practices for evaluating therapeutics in PD animal models.
Preformed Fibrils - A Guide to Cell and Animal Models
An overview of preformed fibril-induced cell & animal models for preclinical testing of disease-modifying therapies across multiple neurodegenerative diseases.
Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease
How α-synuclein influences microglia and astrocytes in Parkinson’s disease and other synucleinopathies.
AAV α-Synuclein Models for Parkinson's Disease Drug Development
Overview of adeno-associated virus (AAV) induced α-synuclein expression in mouse & rat models for use in preclinical studies of disease-modifying therapeutics.
Neurofilament Light Chain in Parkinson's Disease Models
How neurofilament light chain (NfL; NF-L) levels can be used as blood (plasma; serum) & CSF biomarkers in Parkinson's disease mouse and rat models.
Microglial Activation in an α-Synuclein PFF Mouse Model
We have quantified microglial activation, based on morphology, in an α-synuclein preformed fibril (PFF) seeding & spreading mouse model of Parkinson’s disease.
Mitophagy and Parkinson’s Disease
An overview of how impaired mitophagy can lead to neurodegeneration in Parkinson’s disease.
Autophagy, Parkinson's Disease, and Dopaminergic Neurons
An overview of how impaired autophagy can lead to pathologic changes and neurodegeneration in dopaminergic neurons in Parkinson’s disease.
Mitochondrial Dysfunction & Parkinson's Disease
An overview of how mitochondrial dysfunction is associated with neurodegeneration in Parkinson’s disease.
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.
Microglia-Neuron Interactions & Neurodegenerative Diseases
A concise review of the direct interactions between microglia & neurons, and how these cell-to-cell interactions may be affected in neurodegenerative diseases.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
TNF-α (TNF-alpha) & Microglia in Neurodegenerative Diseases
An overview of the function of tumor necrosis factor-alpha (TNF-α) in microglia and its contribution to the progression of neurodegeneration.