Multiplex Immunofluorescence of Brain Sections from the “Low Dox” TDP-43ΔNLS Mouse Model of ALS
Biospective’s TDP-43 mouse models accelerate ALS drug development with translational TDP-43 pathology for preclinical research. As a global neuroscience CRO with extensive experience with the rNLS8 (TDP-43ΔNLS) transgenic mouse model (including our proprietary, slower progressing "Low Dox" model), we offer end-to-end in vivo services — including therapeutic efficacy, mechanism-of-action, and target engagement — backed by clinically relevant biomarkers and quantitative multiplex immunofluorescence.
While several transgenic (tg) mouse models of amyotrophic lateral sclerosis (ALS; also called motor neuron disease [MND]) with TDP-43 aggregation exist, they each have their respective strengths and weaknesses. (Learn more about animal models of ALS in our Resource - A Guide to ALS Models for Drug Discovery.) In our robust ALS transgenic mouse model (often referred to as rNLS8, ΔNLS, delta NLS, or dNLS), human TDP-43 (TARDBP) with a mutated nuclear localization signal (NLS) is overexpressed, resulting in cytoplasmic mislocalization. This mouse model reliably demonstrates phosphorylated TDP-43 aggregates, neurodegeneration, neuroinflammation (activated microglia and astrocytes), muscle wasting, and motor deficits reminiscent of human Amyotrophic Lateral Sclerosis.
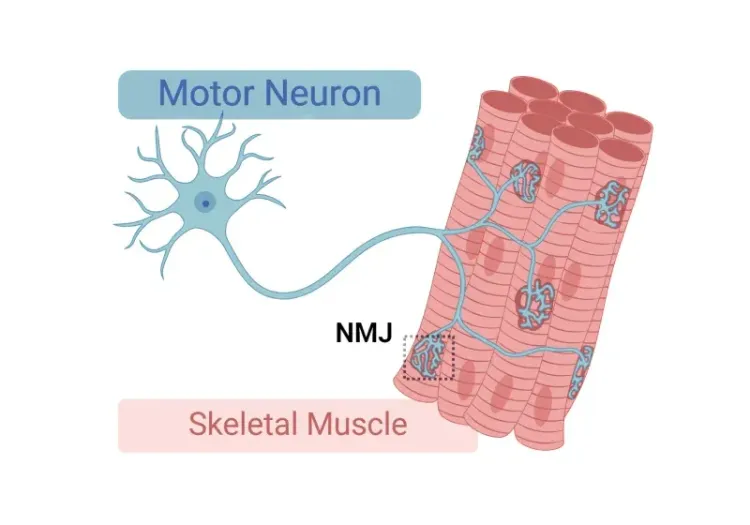
At Biospective, our transgenic TDP-43 mouse models are purpose-built to advance ALS preclinical programs with translational relevance - see TDP-43 ΔNLS (rNLS8) Mice for Drug Development. With unmatched expertise and our in-house colony of the extensively characterized, less aggressive Low Dox rNLS8 model, which has been specifically optimized to better observe drug effects, we provide comprehensive in vivo services, including efficacy testing, biodistribution, mechanism-of-action studies, PK/PD, and target engagement. We have rigorously validated measures of motor impairment, muscle weakness, muscle atrophy, muscle CMAP, neurofilament light chain (NfL) in plasma & CSF, quantitative IHC & multiplex immunofluorescence (including NMJ denervation analysis) for this ALS model to generate decision-ready data for biotech and pharmaceutical partners worldwide.
Overview of the rNLS8 TDP-43ΔNLS Model of ALS
An inducible TDP-43 animal model of ALS tailored for preclinical drug development.
In this mouse model, human TDP-43 with a defective NLS is expressed under the neurofilament heavy (NEFH) promoter. The conventional "Off Dox" model is a rapidly progressing model with death occurring within several weeks of disease induction. Biospective has also developed and extensively characterized a slower progressing (months), less aggressive version of this model (called the "Low Dox" model), which is ideally suited for the preclinical evaluation of therapeutic agents. This approach drives toxic TDP-43 protein expression, triggering a cascade of ALS-like pathology, recapitulating key hallmarks of human disease, including:
-
Cytoplasmic TD aggregates: Cytoplasmic TDP-43 aggregates are a hallmark of familial and sporadic ALS. This model demonstrates accumulation of pathogenic TDP-43 (including phosphorylated TDP-43), forming intracellular inclusions in both the brain and spinal cord.
-
Neurodegeneration: Selective vulnerability of specific neuronal populations (e.g. upper and lower motor neurons) reminiscent of human disease.
-
Robust neuroinflammation: Pronounced activation of microglia and reactive astrocytes in areas of TDP-43 pathology, mirroring the neuroinflammatory response observed in ALS.
- Muscle weakness & atrophy: Decreased grip strength along with reduced CMAP amplitude & increased latency compared to control mice.
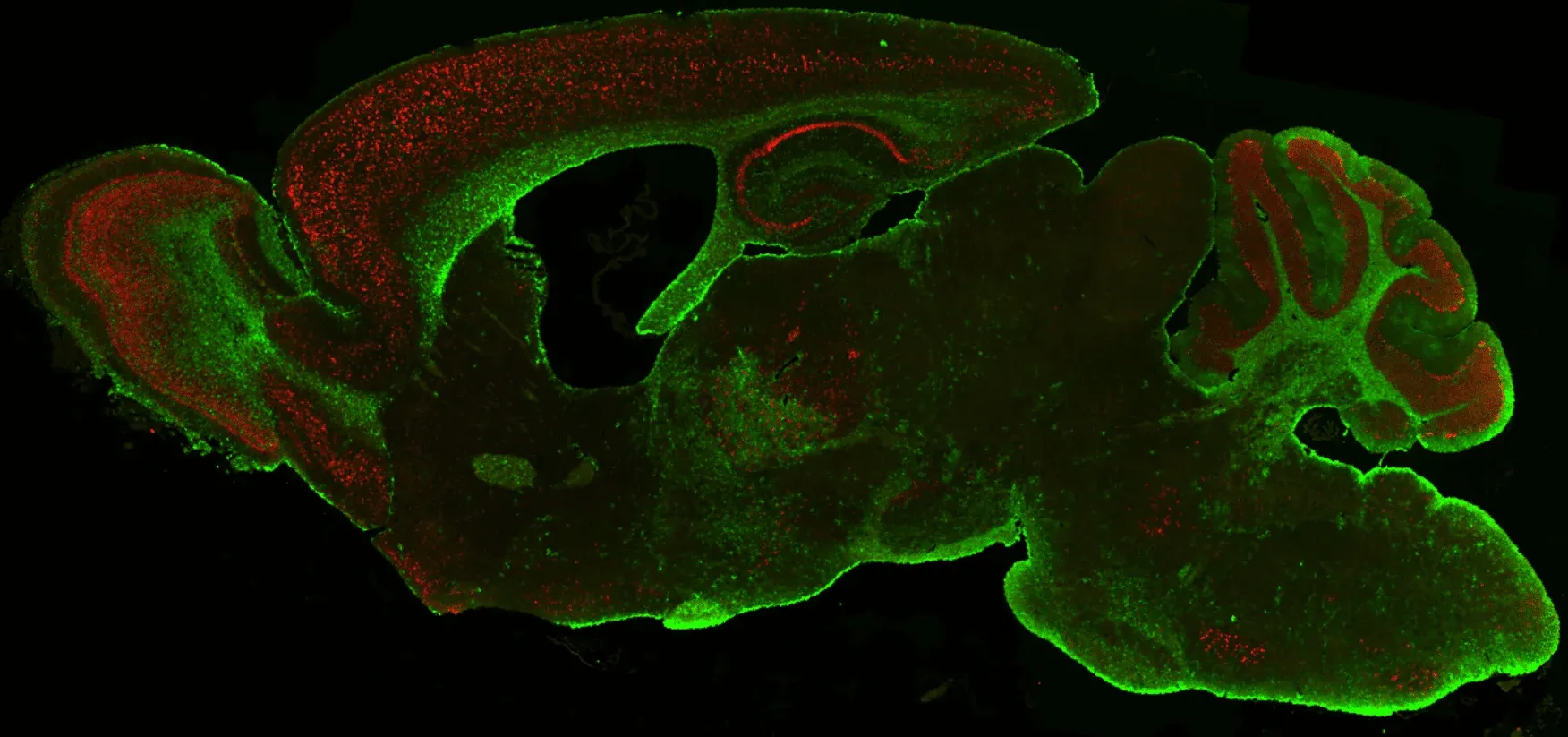
TDP-43 (red) and astrocytes (GFAP; green) in Biospective's Low Dox TDP-43ΔNLS ALS mouse model.
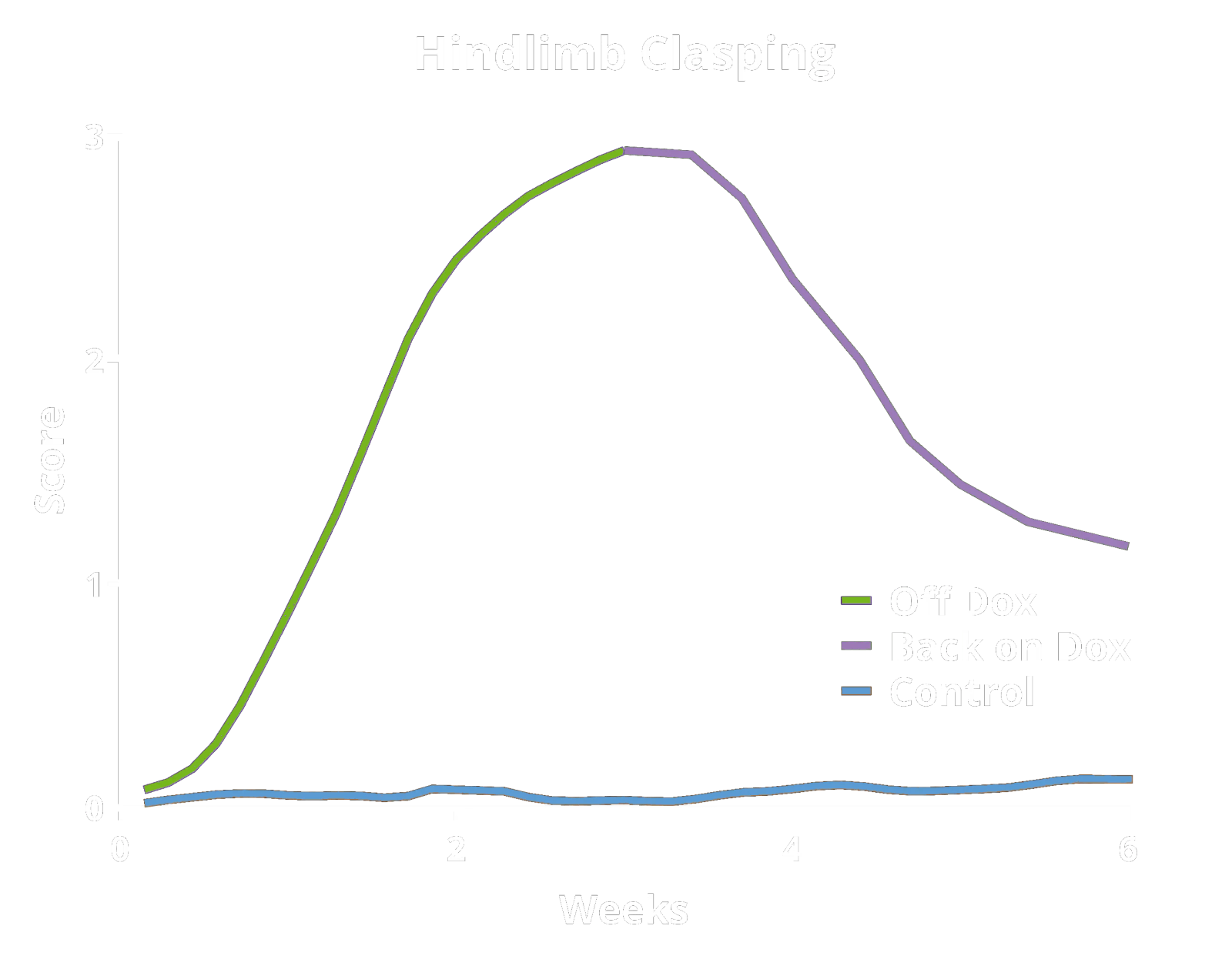
By reproducing clinically relevant pathology, this mouse model provides a disease-relevant platform to evaluate therapeutic interventions under conditions that mirror the clinical hallmarks of human Amyotrophic Lateral Sclerosis. A unique feature of this model is reversibility with a functional and pathologic recovery when Dox is reinstated, demonstrating that is it amenable to disease modification.
Biospective's TDP-43 Model Expertise and Services
Biospective is a global neuroscience CRO with deep expertise in ALS animal models – particularly the Low Dox TDP-43 model, which is a core part of our service portfolio.
We have spent nearly a decade developing and executing studies in ALS models, giving us unparalleled insights into their nuances and optimal use in drug development. We developed the Low Dox model and are the only CRO that provides this model specifically tailored for drug development studies. This depth of experience, combined with our scientific rigor, makes us an ideal partner for outsourcing ALS therapy evaluations. Our team works as an extension of your own, ensuring robust study design and translational relevance at every step.
Some key advantages of partnering with Biospective for TDP-43 ALS model studies studies include:
-
Extensive Experience & Model Characterization: We have extensively characterized both the "Off Dox" and "Low Dox" rNLS8 mouse models through numerous studies over many years, generating datasets that inform best practices and enhance reproducibility. This track record underscores our unique expertise with this ALS model.
-
In-House rNLS8 Transgenic Colony: We maintain a large, in-licensed breeding colony of rNLS8 transgenic mice on-site. Ready access to this colony allows rapid study start-up and large-scale studies (>100 mice) without supply bottlenecks. Monogenic littermates or wild-type controls are also available for well-controlled experimental designs.
-
End-to-End Preclinical Services: Biospective provides integrated services from study design through execution and data analysis. Our capabilities include comprehensive in-life assessments (behavioral testing, motor function assays, etc.), neuroimaging (MRI, CT, PET, SPECT), bioanalysis (fluid biomarkers), IHC & multiplex immunofluorescence), and expert data interpretation. This one-stop approach ensures consistency and accelerates timelines.
-
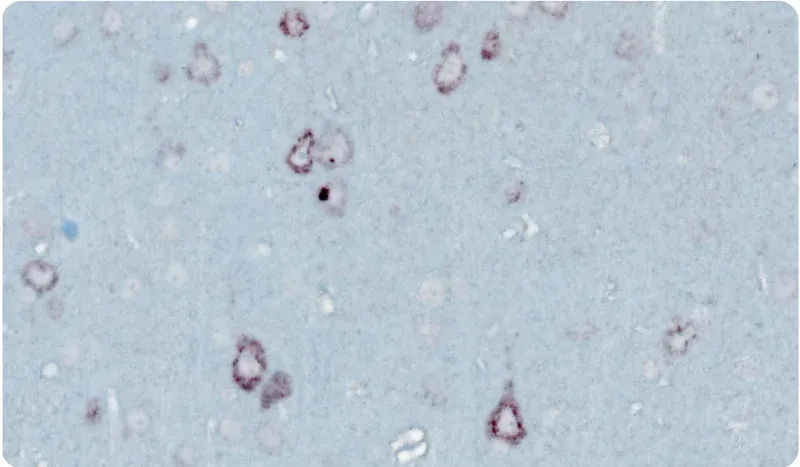
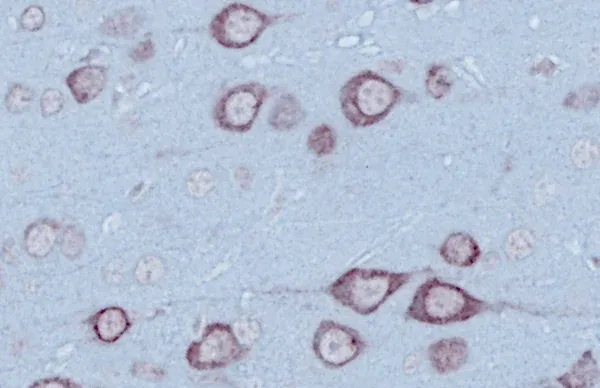
Translational Biomarkers & Readouts: We incorporate translational endpoints that bridge preclinical findings to clinical outcomes. For example, we measure neurofilament light chain (NfL) levels in plasma and CSF as a biomarker of neurodegeneration in this model, analogous to what is seen in patients. We also perform MRI brain imaging to quantify neurodegenerative atrophy in this model, and we use quantitative immunohistochemistry (e.g. human TDP-43, pTDP-43 [p409/410], GFAP astrocyte marker, Iba1 microglial marker) and multiplex immunofluorescence to assess pathology (such as NMJ denervation in this model) and neuroinflammation at the tissue level. These biomarkers and imaging readouts enhance the translatability of study results to human trials.
-
Global Collaboration & Flexibility: We are a global preclinical neuroscience CRO serving biotech and pharmaceutical clients worldwide. Our scientists collaborate closely with sponsors to tailor studies to specific therapeutic mechanisms or targets. We can accommodate custom endpoints or novel treatment paradigms. We also offer flexibility in study design to meet your program’s needs. Importantly, we prioritize scientific rigor, reproducibility, and open communication throughout the partnership.
By leveraging these strengths, Biospective enables biotech and pharma teams to generate decision-quality data in this TDP-43ΔNLS model efficiently. We pride ourselves on fast project initiation, clear data reporting, and supporting our clients across the preclinical phases of drug development.
TDP-43 Model Generation & Study Timelines
Our in-house colony of rNLS8 mice allows us to accommodate pilot to large-scale studies.
rNLS8 (NEFH-hTDP-43-ΔNLS) double transgenic ALS mice ("TDP43 mouse model") are generated by breeding mice having the NEFH-tTA transgene with mice having the tetO-hTDP-43-ΔNLS transgene. This TARDBP model was originally developed and reported by Walker et al. (Acta. Neuropathol., 130: 643-670, 2015). It is a model of amyotrophic lateral sclerosis (ALS) or motor neuron disease (MND). It can also be used as a TDP-43 pathology model of frontotemporal dementia (FTD) or frontotemporal lobar degeneration (FTLD).
These TDP-43 transgenic mice are maintained on a Dox diet during breeding and the initial aging period (typically ~10 weeks-of-age). The mice are then changed from a Dox diet to a standard diet ("Off Dox" model) or an alternate protocol developed by Biospective ("Low Dox" model) to allow for human TDP-43 expression. This regulatable onset allows for precise control over the starting point of the study. A valuable and unique feature of this model is that pathologic and functional recovery (disease reversal) can be achieved by reinstating the Dox diet.
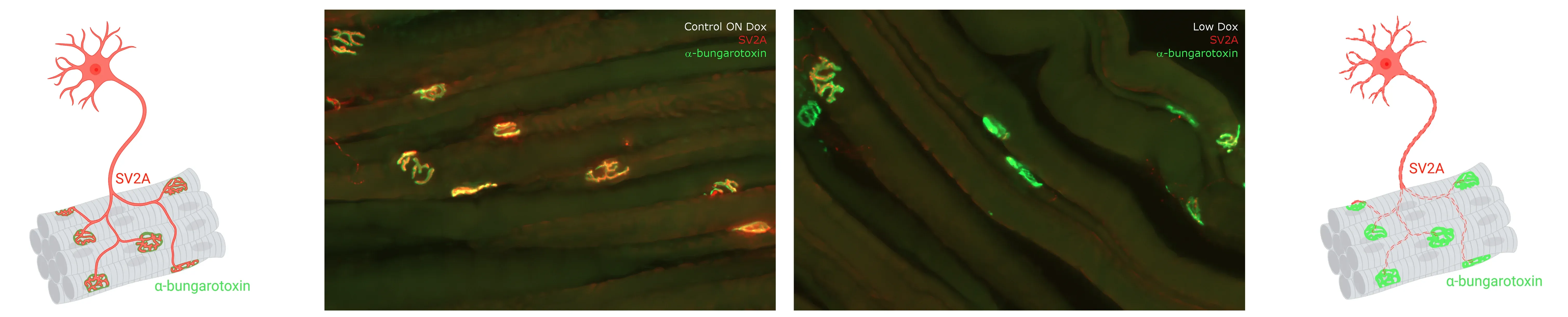
Multiplex immunofluorescence of innervated and denervated neuromuscular junctions (NMJs) from the gastrocnemius muscle of control (left) and Low Dox rNLS8 (right) mice. See our Interactive Presentation — NMJ Denervation in the TDP-43ΔNLS (rNLS8) Mouse Model of ALS — for more details.
Validated Endpoints & Translational Biomarkers
Biospective has implemented a suite of validated endpoints and ALS relevant biomarkers to enable clinical advancement of therapeutic programs.
To fully characterize rNLS8 mice and assess treatment outcomes, Biospective has validated a broad spectrum of endpoints – encompassing behavioral assays, fluid biomarkers, neuroimaging, and histopathology. This comprehensive approach yields robust, quantitative readouts for both efficacy and mechanism-of-action in preclinical studies. Key validated endpoints in our TDP-43dNLS model include:
Behavioral & Functional Endpoints
-
Hindlimb Clasping Test: A sensitive indicator of neurodegeneration (brainstem/spinal reflex integrity) often observed as disease progresses.
-
Grill Agility Test: Provides insight into the animal’s balance, agility, and fine motor skills.
-
Grip Strength Test: Uses a grid or a bar attached to a force-sensing device (grip strength meter) to measure the maximum force exerted by the animal before losing its grip as it is pulled away from the instrument.
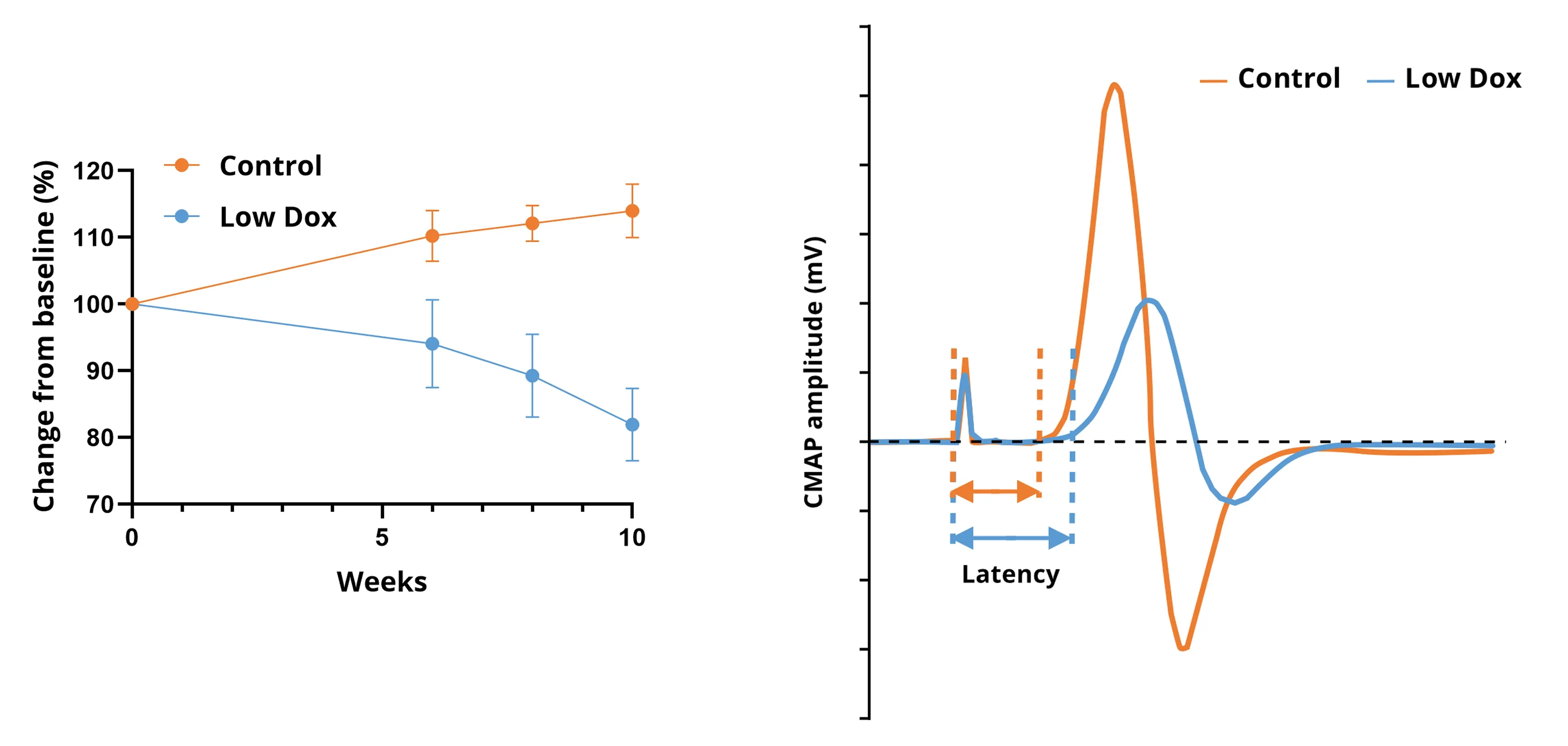
Progressive muscle atrophy of the hindlimb muscles in Low Dox rNLS8 mice measured by non-invasive, in vivo CT imaging (left); and CMAP waveforms from Low Dox and control mice (right).
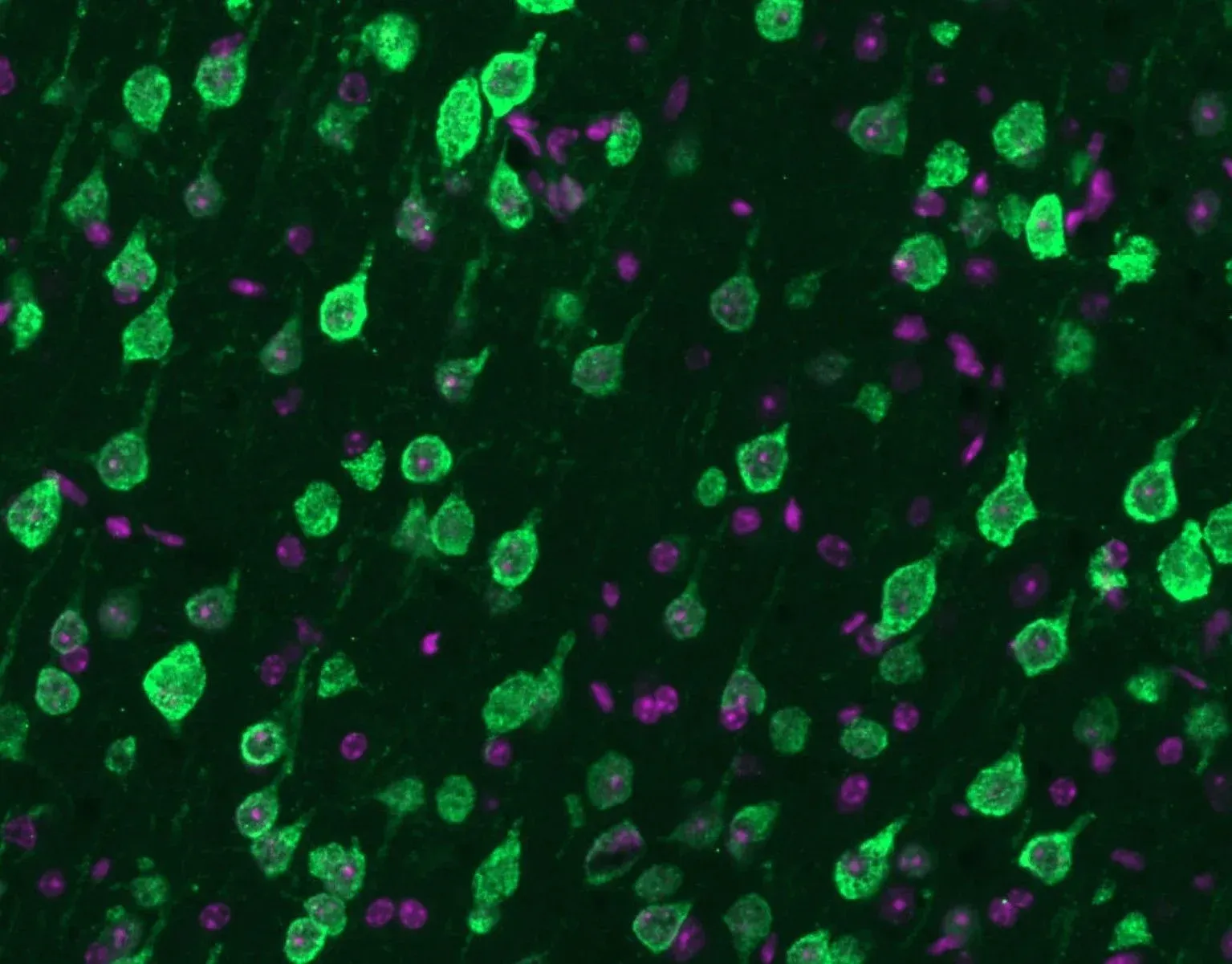
Mislocalization of TDP-43 (green) from the nucleus to the cytoplasm along with reactive astrocytes (violet).
This animation shows regional cortical thinning (green & yellow colors) measured by in vivo MRI in Low Dox rNLS8 mice.
Fluid, Imaging & Tissue Biomarkers
- CSF & Plasma Neurofilament Light Chain (NfL): A fluid biomarker of axonal damage and neurodegeneration, measured in cerebrospinal fluid (and optionally blood plasma). Elevated NfL levels indicate ongoing neuronal injury; this biomarker is also used in clinical trials, making it a valuable bridge between preclinical and clinical results.
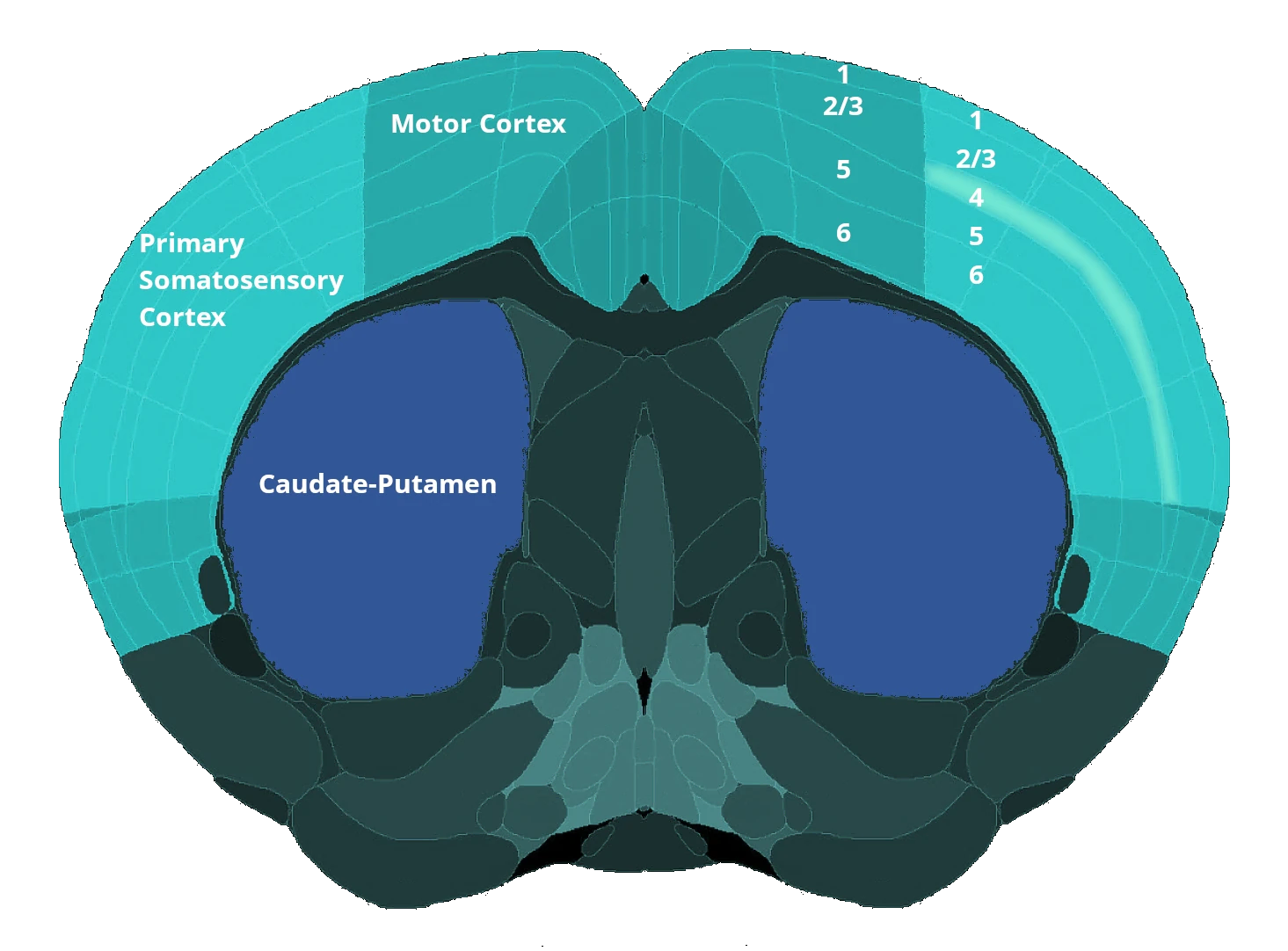
- MRI Brain Atrophy: In vivo magnetic resonance imaging to quantify regional brain volume loss (neurodegeneration) over time. Progressive MRI-detected atrophy in the motor cortex serves as a translational endpoint paralleling human ALS.
- CT Muscle Atrophy: Longitudinal in vivo computed tomography imaging of the hindlimbs coupled with automated image analysis to measure the muscle volumes.
- Muscle Electrophysiology: EMG recordings from the gastrocnemius muscle following electrical stimulation of the sciatic nerve to measure the Compound Muscle Action Potential (CMAP).
- Neuromuscular Junction (NMJ) Innervation: Quantitative multiplex immunofluorescence of the hindlimb muscles to measure the spatial relationship between the presynaptic motor axon terminal and the motor endplate.
- Quantitative Histopathology (IHC/mIF) of Brain & Spinal Cord: High-resolution tissue analyses to quantify ALS-related pathology. We perform immunohistochemistry (IHC) and multiplex immunofluorescence for markers such as phosphorylated TDP-43 (p409/410), activated microglia (Iba1), and astrocytes (GFAP). Digital image analysis of these stained tissues provides quantitative measures of TDP-43 aggregates and neuroinflammation in the brain and spinal cord.
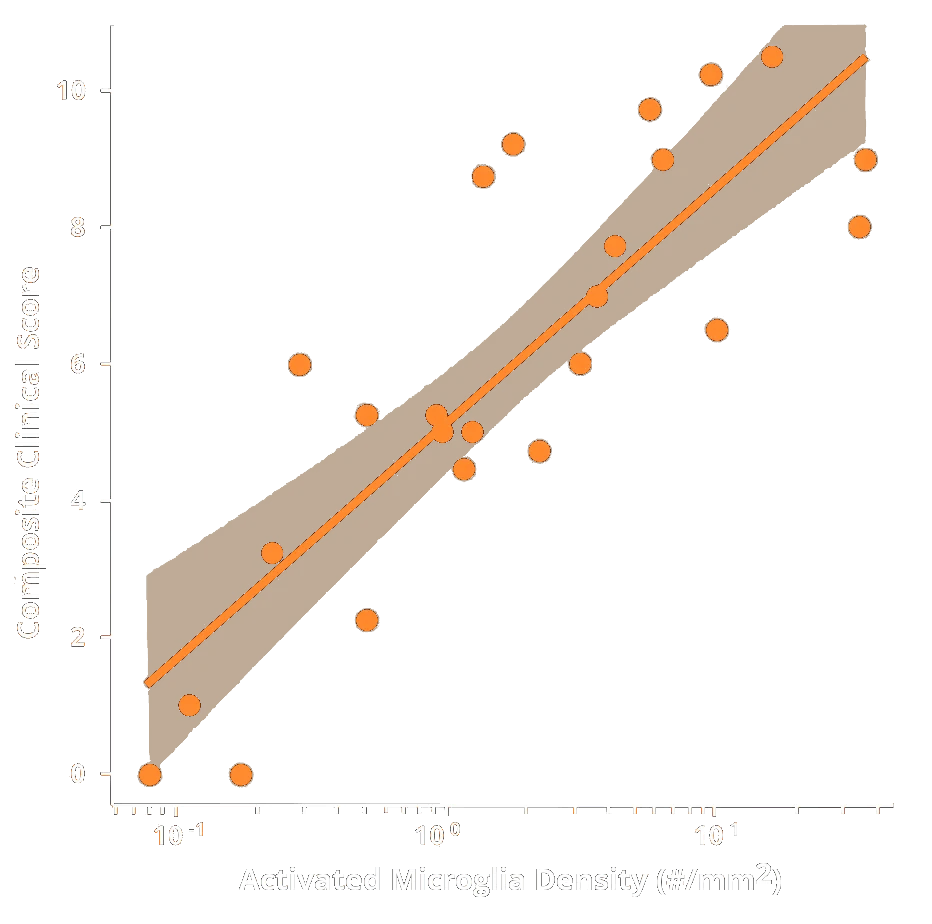
These endpoints span multiple domains – behavioral, imaging, biochemical, and histological – providing complementary measures of disease severity and therapeutic impact. Notably, the inclusion of translational biomarkers like MRI volumetry and NfL helps bridge preclinical findings to the clinic. Neurofilament light (NfL) is a well-established marker of neurodegeneration: when neurons are damaged, NfL is released into CSF and blood, serving as a sensitive indicator of axonal injury and neurodegeneration. In clinical studies, elevated NfL levels correlate with disease progression in various neurological disorders, including ALS. Within our rNLS8 model studies, we observe a similar pattern – as neurons & axons degenerate, CSF & plasma NfL levels rise in parallel with MRI-detected brain atrophy. This mirrored trend underscores the predictive, translational value of our readouts. By tracking such biomarkers longitudinally in vivo, we can quantitatively monitor disease progression and detect therapeutic effects in a way that is directly relatable to patient outcomes.
In addition to these outcome measures, Biospective distinguishes itself by offering seamless end-to-end integration of all study components. We handle every aspect of the experiment – from model induction, longitudinal behavioral testing, and in vivo MRI/CT imaging to biofluid collection and post-mortem tissue analysis. Our scientific team employs advanced analytics (including automated image analysis for TDP-43 inclusions and AI-driven cell morphology classification) to extract rich datasets from the model. All data are rigorously analyzed and integrated into an interpretable report, allowing you to make informed decisions on your therapeutic candidate’s performance.
Interactive Microscopy Images
Use the Image Viewer below to navigate through high-resolution microscopy images via the left-hand panel or the on-screen arrows. You can pan around the images with your mouse, and zoom in/out using the scroll wheel or the +/- controls. The Control Panel (top-right) allows toggling of image channels and segmentation overlays. For the best experience, we recommend switching to full-screen mode.
Multiplex immunofluorescence tissue sections that demonstrate cytoplasmic human TDP-43, reactive astrocytes, and activated microglia in Biospective's "Low Dox" rNLS8 ALS mouse model.
Click to copy link
This comprehensive capability means that whether you aim to measure drug biodistribution, target engagement (e.g. TDP-43 clearance), axonal integrity, and/or neuroinflammatory modulation, our team can incorporate the appropriate assays and analyses into the study design. All data are rigorously analyzed and integrated into an interpretable package, allowing you to make informed decisions on your therapeutic candidate’s performance.
Leverage Biospective's TDP-43 Models for ALS Drug Development
By partnering with Biospective for your ALS research, you gain access to an internationally recognized team of neurobiology experts and a deeply characterized preclinical model that can accelerate your drug development pipeline.
We have extensive experience executing studies in the rNLS8 TDP-43 mouse model – from exploratory proof-of-concept efficacy studies to detailed mechanistic investigations – across a range of therapeutic modalities (small molecules, biologics, antibodies, gene therapies, antisense oligonucleotides, etc.). Our commitment to scientific rigor and translational relevance is reflected in the quality of our data and our continuous innovation in model validation. As a full-service CRO, we integrate study design, execution, analysis, and reporting, ensuring that your ALS therapeutic candidates are evaluated with the highest level of expertise and care.
Learn more about our characterization of this model, our validated measures, and our Preclinical Neuroscience CRO services.
Related Content
Up-to-date information on ALS and best practices related to the evaluation of therapeutic agents in ALS animal models.
Neuromuscular Junction (NMJ) Morphology & ALS Models
Insights into neuromuscular junction (NMJ), its role in amyotrophic lateral sclerosis (ALS), and tools & methods used to study morphological changes in NMJs.
ALS Mouse Models & Spinal Motor Neurons
An overview of the involvement of spinal motor neurons in disease progression in mouse models of Amyotrophic Lateral Sclerosis (ALS).
A Guide to ALS Models for Drug Discovery
A Resource for the most effective use of research animal models (mouse & rat models) of Amyotrophic Lateral Sclerosis (ALS) for preclinical testing of therapeutics.
TDP-43 ΔNLS (rNLS8) Mice for ALS Drug Development
This resource provides information about the use of the ΔNLS (deltaNLS, hTDP-43ΔNLS, hTDP-43DeltaNLS, dNLS, TDP43 NLS, rNLS8) TDP-43 transgenic mouse model of ALS for preclinical therapeutic studies.
Microglia Morphology in ALS, Alzheimer's Disease & Parkinson's Disease
An overview of microglial morphological analysis and the applications to neurodegenerative disease research and drug discovery & development.
Autophagy & Neurodegenerative Diseases
An overview of how cellular autophagy plays a role in brain health and neurodegeneration.
Autophagy and Transcription Factor EB (TFEB)
An overview of Transcription Factor EB (TFEB) and its role in autophagy and neurodegenerative diseases.
TDP-43: Role in ALS and Frontotemporal Dementia (FTD)
An overview of TDP-43, its physiological role, significance in ALS and FTD pathology, and therapeutic strategies involving TDP-43.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
TNF-α (TNF-alpha) & Microglia in Neurodegenerative Diseases
An overview of the function of tumor necrosis factor-alpha (TNF-α) in microglia and its contribution to the progression of neurodegeneration.
Microglial Senescence and Neurodegenerative Diseases
An overview of microglial senescence and its role in neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD).
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.
TNF-α & (TNF-alpha) Astrocytes in Neurodegenerative Diseases
An overview of TNF-α signaling in astrocytes, its role in neurodegeneration, and therapeutic strategies targeting this pathway..
Microglia-Neuron Interactions & Neurodegenerative Diseases
A concise review of the direct interactions between microglia & neurons, and how these cell-to-cell interactions may be affected in neurodegenerative diseases.