What is the NLRP3 inflammasome?
The NOD-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome, the most extensively studied inflammasome, is a multi-protein complex that plays a critical role in regulating the innate immune system and inflammatory signaling, and is involved in the development of various immune and inflammation-related diseases. Its canonical activation pathway is triggered when either pathogen-associated molecular patterns (PAMPs) or host-derived damage-associated molecular patterns (DAMPs), released in response to injury or cellular stress, are detected by pattern recognition receptors (PRRs). NOD-like receptors (NLRs), toll-like receptors (TLRs), and absent in melanoma 2-like receptors (ALRs) are distinct types of PRRs that recognize PAMPs and DAMPs and prime the cell for the subsequent activation of the NLRP3 inflammasome.
See: What is an Inflammasome? & What is NLR3?
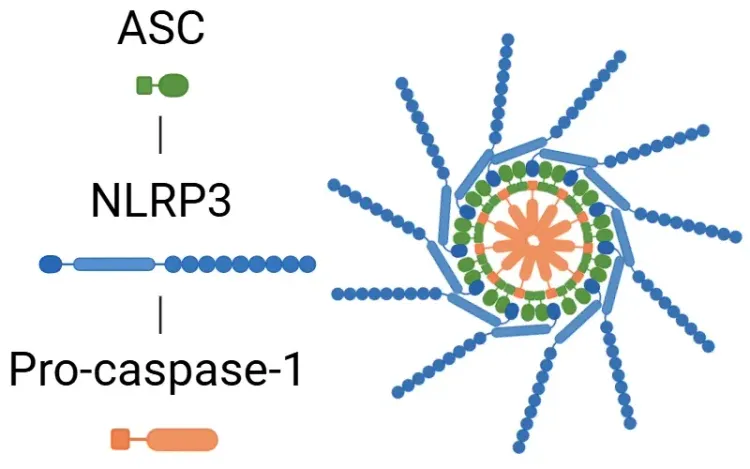
The NLRP3 inflammasome is composed of three main components:
- NLRP3 - acts as the sensor
- ASC (apoptosis-associated speck-like protein containing a CARD) - functions as the adaptor
- Pro-caspase-1 - serves as the effector.
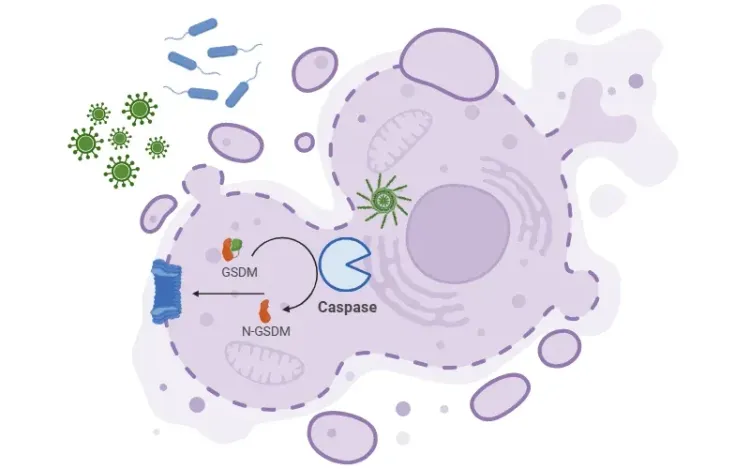
The formation of the inflammasome process begins with the detection of PAMPs or DAMPS. ASC then binds to pro-caspase-1, converting it into active caspase-1. In turn, active caspase-1 cleaves pro-interleukin (IL)-1β and pro-IL-18 into their active forms, IL-1β and IL-18. Caspase-1 also activates gasdermin D (GSDMD), which forms membrane pores, enabling the release of these proinflammatory cytokines, and serves as a trigger of pyroptosis.
Key outcomes of NLRP3 activation include:
- Activation of caspase-1
- Cleavage and release of IL-1β and IL-18
- Activation of gasdermin D (GSDMD), forming membrane forms
- Induction of pyroptosis (a form of inflammatory cell death)
See: What is IL-1β (Interleukin-1 Beta)? and What is Pyroptosis?
In addition to immune and inflammation-related diseases, the NLRP3 inflammasome has also been implicated in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) (Holbrook, 2021; Singh, 2023). While microglia and astrocytes initially provide protective immune responses in the CNS, their chronic activation can lead to the spread of neuroinflammation, which in turn contributes to the progression of these neurodegenerative diseases (Wang, 2024). As such, therapies targeting the NLRP3 inflammasome are gaining attention for their potential to reduce chronic inflammation and alleviate disease symptoms.
Recent studies exploring small molecules that modulate NLRP3 activation have shown encouraging results in blocking chronic inflammation and improving disease outcomes (Blevins, 2022). While there are still challenges in developing effective treatments targeting the NLRP3 inflammasome for CNS diseases, such as bypassing the blood-brain barrier (BBB), continued advancements in drug development and targeted therapies will pave the way for effective treatments to come.
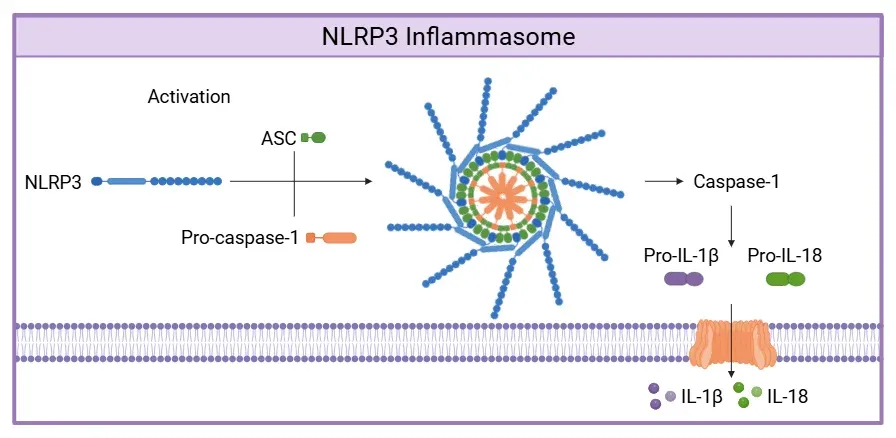
Canonical Activation of the NLRP3 Inflammasome.
NLRP3 senses PAMPs or DAMPs, recruiting of ASC and pro-caspase-1 to form the inflammasome complex. Active caspase-1 cleaves pro-IL-1β and pro-IL-18 into their active cytokines and processes GSDMD, whose pores enable cytokine release. Figure adapted from Wang et al. (Wang, 2024) under the Creative Commons Attribution License.
What is the role of the NLRP3 inflammasome in AD, PD, and ALS?
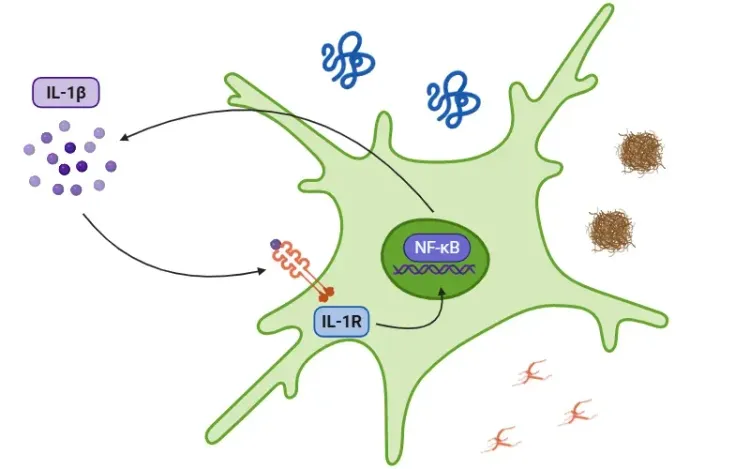
Neurodegenerative diseases, including AD, PD, and ALS, are becoming increasingly prevalent. These disorders are characterized by the progressive accumulation of misfolded proteins in various regions of the CNS, leading to neuronal loss. A critical consequence of this protein accumulation is the activation of the NLRP3 inflammasome, which triggers the release of proinflammatory cytokines IL-1β and IL-18, contributing to neuroinflammation. Growing evidence underscores the central role of inflammasomes in disease progression, amplifying inflammation and neuronal damage.
For more on the role of IL-1β and neurodegenerative diseases, see: Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
Alzheimer’s Disease (AD)
AD is a progressive neurodegenerative disorder marked by cognitive decline, memory impairment, and changes in behavior and mood. The accumulation of Aβ plaques and hyperphosphorylated tau neurofibrillary tangles is central to AD pathogenesis. However, neuroinflammation also plays a significant role in disease progression. In AD, Aβ and tau act as DAMPs, activating the NLRP3 inflammasome, particularly in microglia. This activation leads to the release of proinflammatory cytokines like IL-1β, exacerbating neuroinflammation and accelerating neurodegenerative (Wang, 2024). Accordingly, peripheral immune cells and postmortem brain tissue from AD patients show elevated levels of IL-1β and IL-18, along with increased expression of inflammasome components NLRP3, ASC, and caspase-1, with ASC expression correlating with levels of Aβ and tau (Heneka, 2013; Saresella, 2016; Vontell, 2023).
Key experimental findings supporting NLRP3's role in AD include:
- In APP/PS1 AD mouse models:
- Increased caspase-1 processing has been observed (Heneka, 2013).
- Crossing these mice with NLRP3 or caspase-1 deficient models preserves memory, suggesting these proteins mediate inflammation linked to cognitive dysfunction (Heneka, 2013).
- Increased Aβ phagocytosis occurs in APP/PS1/NLRP3-/- or APP/PS1/Casp-1-/- mice, indicating that NLRP3 inflammasome activation reduces Aβ learance (Heneka, 2013).
- Regarding tau pathology:
- Tau22 mice show increased caspase-1, ASC, and IL-1β levels (Ising, 2019).
- Crossing Tau22 mice with ASC or NLRP3 deficiency results in lower tau hyperphosphorylation and aggregation, as well as preserved memory (Ising, 2019).
- Injection of APP/PS1 brain homogenate in Tau22 mice induces tau hyperphosphorylation, but this effect is absent in Tau22/ASC-/- or Tau22/NLRP3-/- mice, suggesting NLRP3 is key to the Aβ-tau cascade (Ising, 2019).
Given its key role in both Aβ and tau pathology, targeting the NLRP3 inflammasome is a promising strategy that could yield therapeutic benefits and help slow the progression of AD, as well as other tauopathies (Heneka, 2013; Ising, 2019).
Parkinson’s Disease (PD)
PD, the second most common neurodegenerative disease after AD, is primarily characterized by motor symptoms such as muscle rigidity, bradykinesia, and resting tremor, along with non-motor symptoms, including mood disorders like depression and behavioral disturbances. PD is defined by hallmark features such as the progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) and the accumulation of Lewy bodies, which are primarily composed of α-syn aggregates. In addition to these hallmark features, inflammation also plays a crucial role in PD pathology (Li, 2021). In post-mortem tissue from PD patients, increased levels of ASC and NLRP3 have been detected, suggesting the activation of the NLRP3 inflammasome in this disease (Anderson, 2021).
Key findings from experimental models include:
- MPTP-induced mouse model of PD:
- Crossing with NLRP3 or caspase-1 knockout mice reduces DA neuron loss and improves motor function (Qiao, 2017; Lee, 2019).
- In NLRP3-deficient mice, MPTP does not induce microglial recruitment, IL-1β production, or caspase-1 activation in the SN, suggesting a central role for microglial NLRP3 activation in PD-related neurodegeneration (Lee, 2019).
- A selective dopamine D2 receptor agonist inhibits NLRP3 inflammasome activation in the SN and suppresses caspase-1 and IL-1β expression in cultured astrocytes (Zhu, 2018).
While some studies support NLRP3 inflammasome activation in astrocytes (Freeman, 2017), conflicting evidence exists, highlighting the need for further research.
- α-syn pathology and NLRP3:
- Caspase-1 can directly cleave α-syn (Wang, 2016).
- Blocking NLRP3 activation with the small molecular inhibitor MCC950 reduces α-syn aggregation, dopaminergic degeneration, neuroinflammation, and motor deficits in both the PFF and AAV-Syn mouse models of PD (Gordon, 2018; Grotemeyer, 2023).
These findings suggest that targeting the NLRP3 inflammasome could offer a promising therapeutic approach for PD.
Amyotrophic Lateral Sclerosis (ALS)
ALS is a neurodegenerative disease characterized by the progressive degeneration of motor neurons in the spinal cord, brainstem, and motor cortex, leading to symptoms such as muscle weakness, difficulty speaking and swallowing, and progressive paralysis. ALS patients have mutations in C9orf72, TARDBP, SOD1, and FUS, with TDP-43 and SOD1 proteins being the most extensively studied. These proteins form abnormal aggregates, leading to impaired protein clearance and neuronal dysfunction. Additionally, the NLRP3 inflammasome may play a significant role, as elevated levels of NLRP3, ASC, IL-18, and caspase-1 have been detected in post-mortem tissue of ALS patients (Johann, 2015).
Key experimental findings:
- In ALS mouse models:
- Both SOD1G93A and TDP-43Q331K mice show upregulated expression of NLRP3 inflammasome pathway genes in spinal cord tissue (Deora, 2020).
- In SOD1G93A mice, astrocytes in the spinal cord are the main expressers of NLRP3 components (Johann, 2015).
- Caspase-1 inhibition in the SOD1G93A model delays disease onset, neurological deterioration, and mortality (Zhang, 2013).
- Therapeutic challenges:
- MCC950 treatment does not reduce spinal cord inflammation in the SOD1G93A model (Clénet, 2023).
- This suggests that multiple inflammasomes may be activated in ALS, and targeting NLRP3 alone may not be sufficient.
In conclusion, while NLRP3 inflammasome activation has been extensively studied in AD and PD, its role in ALS remains less well understood. In addition to NLRP3, other inflammasome complexes, such as AIM2, NLRC4 and NLRP1, may also contribute to neuropathology in these neurodegenerative diseases. More preclinical studies and clinical trials are needed to unravel the complex role of inflammasomes in neurodegenerative diseases, identify potential therapeutic targets, and develop effective treatments.
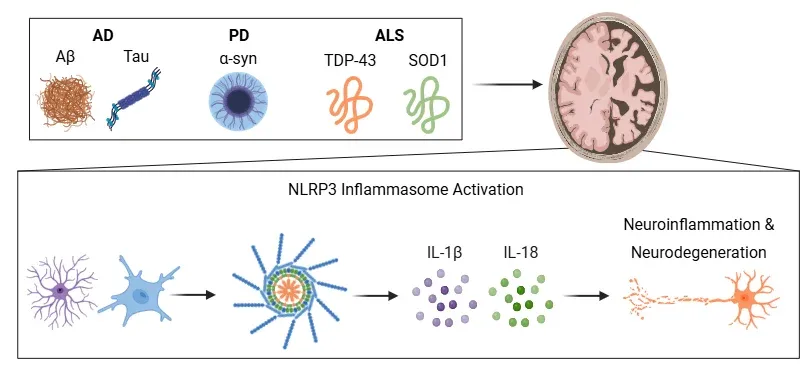
NLRP3 Inflammasome in Neurodegenerative Diseases.
Misfolded protein aggregates in AD, PD, and ALS activate the NLRP3 inflammasome in microglia and astrocytes, promoting IL-1β and IL-18 release. These cytokines drive neuroinflammation and neurodegeneration. Figure adapted from Wang et al. (Wang, 2024) under the Creative Commons Attribution License.
What strategies are being explored for therapeutic targeting of the NLRP3 inflammasome pathway in neurodegenerative diseases?
The NLRP3 inflammasome plays a critical role in neurodegenerative diseases by driving neuroinflammation, a key contributor to disease progression. This finding has led to the development of various compounds aimed at targeting the NLRP3 inflammasome pathway to reduce neuroinflammation and its detrimental effects.
MCC950 (CRID3):
- One of the most well-known and widely studied NLRP3 inhibitors
- Specifically inhibits NLRP3 inflammasome activation
- Prevents release of proinflammatory cytokines like IL-1β without affecting other inflammasome complexes
- Shows promise in preclinical studies for alleviating symptoms in mouse models of neurodegenerative diseases (Blevins, 2022).
Other NLRP3 inhibitors:
- Glyburide and OLT1177 are under exploration for therapeutic efficacy
- BAY 11-7082 and parthenolide inhibit multiple inflammasome complexes but with less specificity for NLRP3
Targeting components of the NLRP3 inflammasome pathway:
- Caspase-1 inhibitors (VX-740 and VX-765):
- Selectively block release of IL-1β and IL-18, reducing inflammation
- Improve cognitive function in AD mouse models (Wang, 2024).
- ASC inhibitors (IC100):
- Demonstrated anti-inflammatory effects in the experimental autoimmune encephalomyelitis (EAE) model (Wang, 2024).
- GSDMD inhibitors:
- Disulfiram blocks GSDMD pore formation and reduces inflammation in PD cell models
- Necrosulfonamide reduces neuroinflammation and protect DA neurons in the MPTP-induced PD model (Wang, 2024).
Despite promising preclinical results, clinical trials investigating NLRP3 inhibitors for treating neurodegenerative diseases remain limited. One notable study is the Phase 2 trial of Usnoflast (ZYIL1), a small molecule NLRP3 inhibitor tested in ALS patients (NCT05981040). This trial followed a successful Phase 1 study showing significant inhibition of IL-1β and IL-18, highlighting the potential of NLRP3 inhibition as a treatment strategy for neurodegenerative diseases (Parmar, 2023).
A key challenge in developing NLRP3-targeted therapies for CNS diseases is crossing the BBB, which is a significant obstacle. Additionally, blocking IL-1β signaling may increase susceptibility to infections, requiring a balance between therapeutic efficacy and safety. These challenges emphasize the need for small-molecule inhibitors of NLRP3 to overcome pharmacokinetic and selectivity limitations (Blevins, 2022).
In summary, while targeting the NLRP3 inflammasome shows promise for treating neurodegenerative diseases, it requires careful consideration of specificity, BBB penetration, and potential adverse effects. Ongoing research and clinical trials will be crucial in determining the therapeutic viability of these strategies.
Our team would be happy to answer any questions about the NLRP3 inflammasome and neurodegenerative diseases or provide specific information about the AD, ALS, and PD models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on NLRP3 inflammasome and neurodegenerative diseases and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
What is Pyroptosis? | A Drug Development Perspective
An overview of pyroptosis, its role in various diseases, and therapeutic strategies related to pyroptosis pathways.
Inflammasome – A Therapeutic Target for Multiple Diseases
An overview of inflammasomes, including their mechanisms of action, roles in diseases, and targeting for drug development.
What is NLRP3?
An overview of NLRP3 inflammasome activation triggers, disease associations, and therapeutic targeting strategies.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
What Is IL-1β (IL-1b)? Function, Signaling, and Biological Role
An overview of IL-1β, including its signaling pathways, involvement in disease mechanisms, and potential therapeutic targets.
Lysosome Dysfunction in Microglia & Astrocytes
An overview of lysosomal dysfunction in microglia & astrocytes, and its role in neurodegenerative diseases.