What is the AAV α-synuclein (AAV-Syn) model?
The progressive loss of dopaminergic neurons in the substantia nigra pars compacta, accumulation of α-synuclein-rich Lewy bodies, and associated motor dysfunction are hallmark features of Parkinson’s disease (PD). As a key contributor to PD pathophysiology, measurement of pathological α-synuclein is a key readout in preclinical PD models, and has been the rationale for the development of PD mice and rats overexpressing wild-type or mutant α-synuclein through transgenesis, pre-formed fibril (PFF) injection, or viral vector-based somatic transduction techniques.
The stereotaxic injection of adeno-associated virus (AAV) vectors overexpressing human α-synuclein (AAV-Syn) leads to the progressive loss of dopaminergic neurons in the substantia nigra pars compacta and nigrostriatal denervation. Using validated AAV vectors, AAV-Syn injections into the substantia nigra of the mouse or rat brains leads to stable α-synuclein transduction by 3-4 weeks post-injection. Typically, a progressive, greater than 50% loss of dopaminergic neurons in the substantia nigra pars compacta can be observed in these models. Additionally, AAV-Syn overexpression leads to α-synuclein aggregation, neuroinflammation, and a robust motor phenotype.
Microscopy Images
The Interactive Image Viewer below allows you to explore entire immunofluorescence tissue sections.
You can pan around the image using the left mouse button. You can zoom in and out using the mouse/trackpad (up/down) or the + and - buttons in the upper left corner. You can toggle (on/off), change color, and adjust image settings for the channels and segmentations in the Control Panel in the upper right corner. You can change tissue sections using the Section Slider in the Control Panel.
In this visualization, an AAV-A53T α-Syn mouse brain is seen with the Section Slider in the left position and an AAV-null control mouse brain can be seen with the Section Slider in the right position.
There are many α-synuclein-based animal models of Parkinson's disease available. However, there are several reasons why the AAV-Syn model is one of the preferred models for PD drug development. The disease phenotype appears in a relatively short time frame compared to α-synuclein-overexpressing transgenic animal models. AAV-Syn induced mouse and rat models have strong face and construct validity; they are superior in reproducing key molecular aspects that are in line with human PD, including the role of pathologic α-synuclein, progressive time course of dopamine (DA) dysfunction, and a predictable patten of neuroinflammation. Compared to traditional neurotoxin-based PD models (such as 6-hydroxydopamine [6-OHDA] and MPTP), AAV-Syn has improved predictive validity and is more effective at predicting therapeutic responses in human PD.
The AAV-Syn model is well-suited for drug development targeting nigrostriatal degeneration and motor dysfunction. It can also be used to assess experimental therapeutic agents targeting neuroinflammation. At Biospective, our stereotaxic delivery of AAV-Syn overexpressing human mutant A53T α-synuclein mice replicate most of the essential aspects of PD, including α-synuclein aggregation, neurodegeneration, neuroinflammation (microglia & astrocytes), and motor dysfunction.
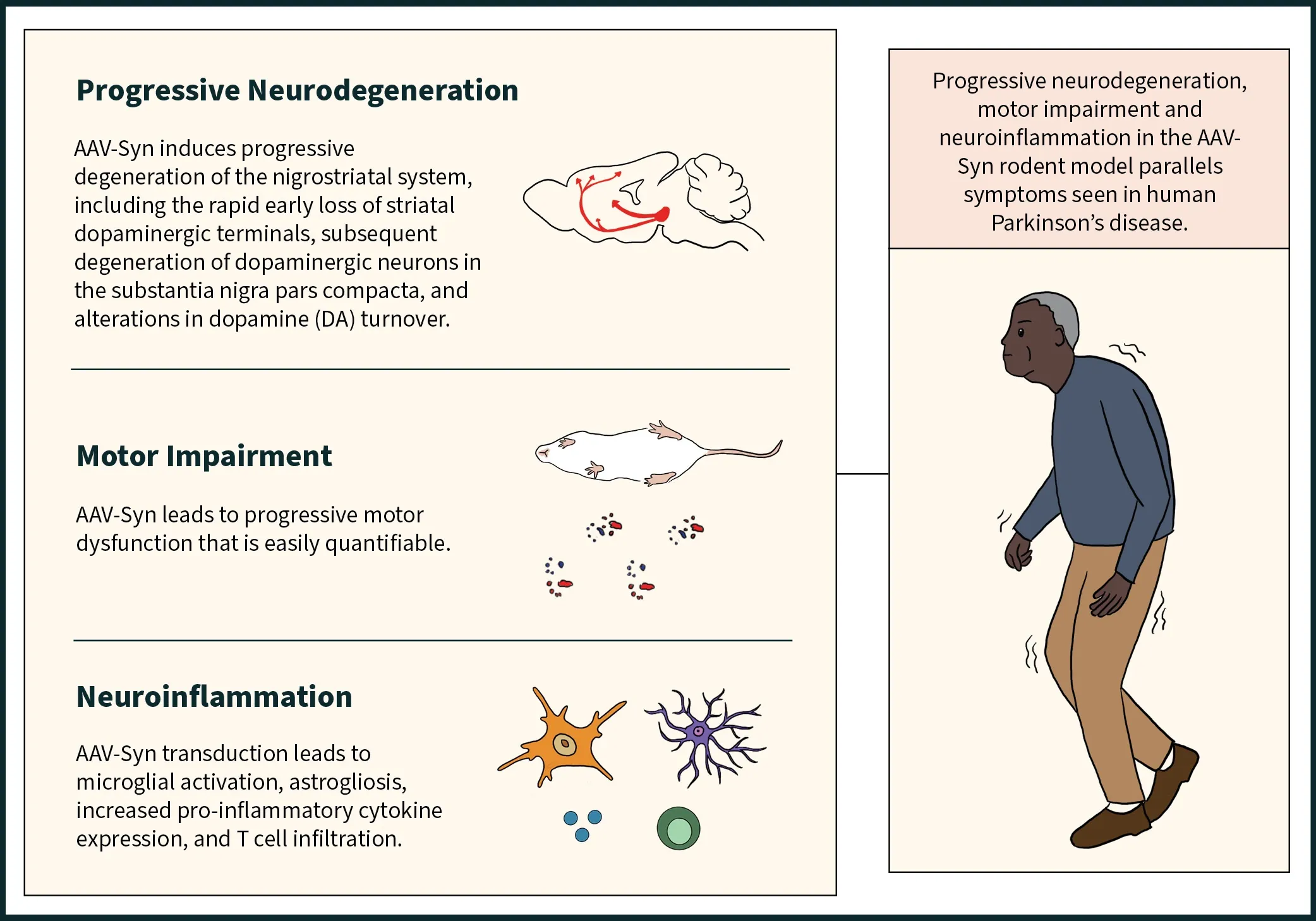
The AAV-Syn model shows progressive neurodegeneration including loss of dopaminergic neurons in the substantia nigra pars compacta and associated denervation of the ipsilateral striatum, motor impairment that can be readily measured by standardized tests, and neuroinflammation including activated microglia, reactive astrocytes, and increased pro-inflammatory cytokine expression.
Click to copy link
Which motor & behavioral phenotypes can be observed in these models?
AAV-Syn models are typically generated by injecting human α-synuclein AAV vectors unilaterally into the substantia nigra of wild-type mice or rats, which induces quantifiable motor dysfunction in many motor and behavioral tests. A few commonly used, robust tests are highlighted below.
Elevated Body Swing Test (EBST) or Tail Suspension Swing Test (TSST)
The EBST has been typically used to evaluate experimental stroke in rodents, and has been adapted for use in unilateral lesion models of PD. Biospective has validated the EBST in the AAV-Syn mouse model, where we have shown significant swing asymmetry after injection of AAVs overexpressing A53T mutant human α-synuclein (AAV-hA53Tα-Syn).
Learn more about the Elevated Body Swing Test (EBST) or Tail Suspension Swing Test (TSST).
Rodents with a unilateral dopaminergic deficit will swing to the contralateral side.
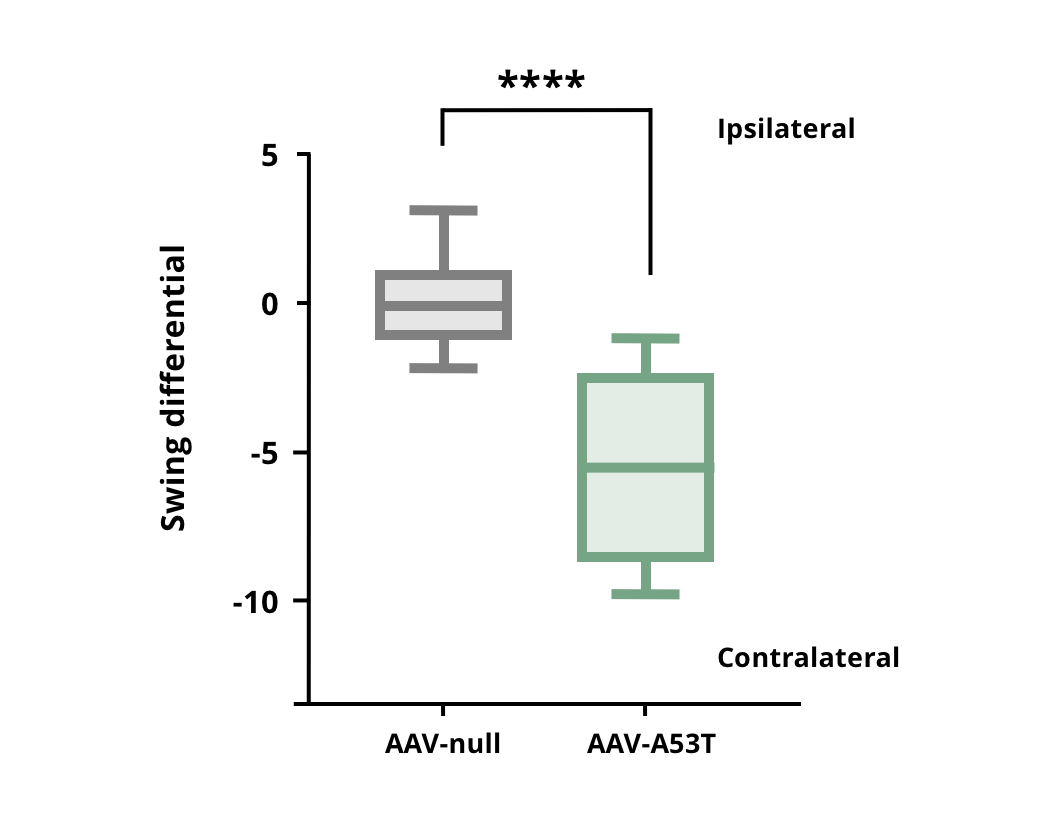
Increased contralateral swings in AAV-hA53Tα-Syn injected mice compared to AAV-null control mice. **** p<0.0001.
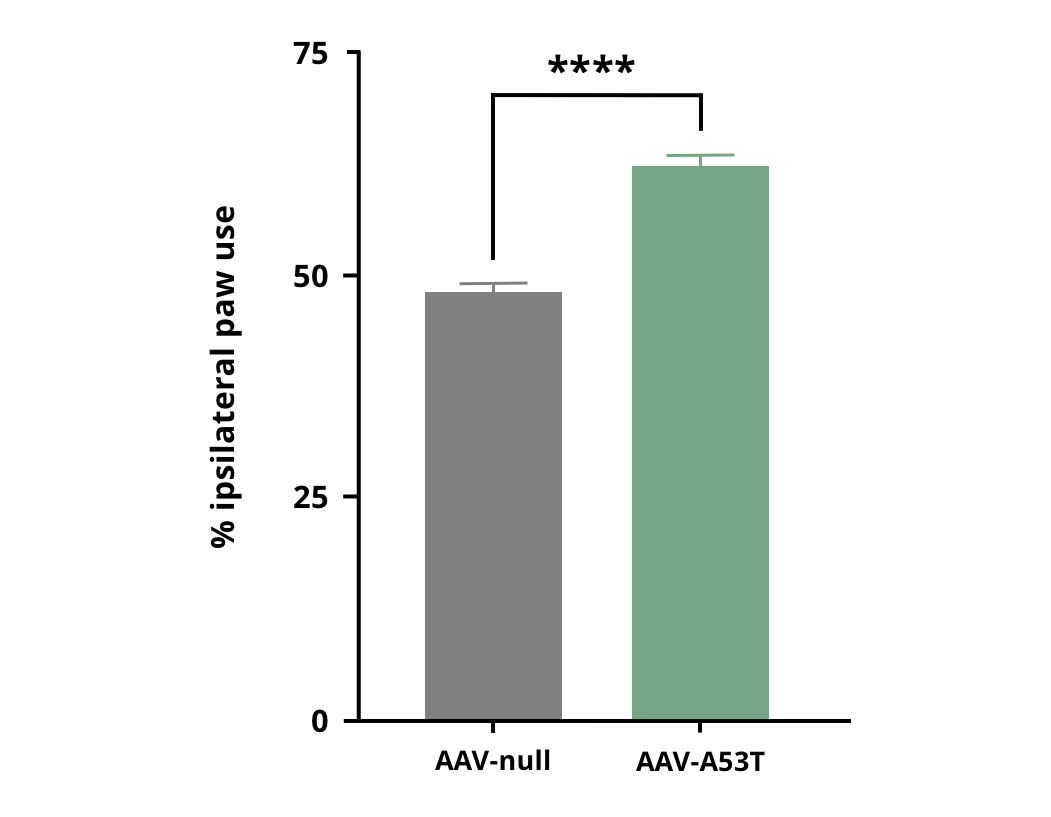
Increased use of the ipsilateral forepaw in AAV-hA53Tα-Syn injected mice compared to AAV-null control mice. **** p<0.0001.
Cylinder Test
Increased forelimb asymmetry is seen as early as 3 weeks post-AAV-Syn injection in the rat and 5 weeks in the mouse.
At Biospective, we routinely use the cylinder test to measure forelimb dysfunction, and we have been able to show significant forelimb deficits several weeks following AAV-hA53Tα-Syn injection.
Learn more about the Cylinder Test.
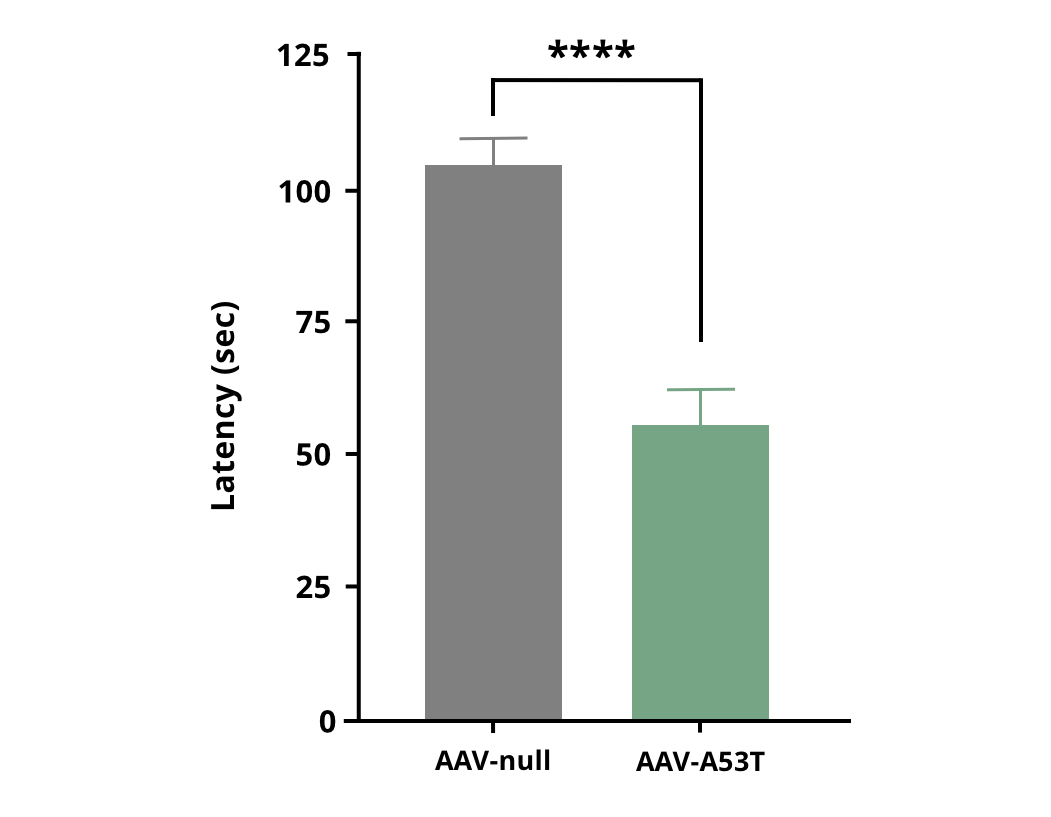
Decreased time spent on the rotarod in AAV-hA53Tα-Syn injected mice compared to AAV-null control mice. **** p<0.0001.
Rotarod Test
The rotarod test measures neuromuscular coordination and balance. Deficits are observed starting at 4 and 5 weeks post AAV-Syn injections in the rat and mouse, respectively.
Similarly, Biospective has been able to demonstrate significant deficits on the accelerating rotarod in AAV-hA53Tα-Syn injected mice.
Learn more about the Rotarod Test.
Amphetamine/Apomorphine-Induced Rotation
Unilaterally-injected AAV-Syn leads to increased rotational behavior following injection of amphetamine or apomorphine in rats starting at 8 weeks post-injection.
Learn more about Amphetamine/Apomorphine-Induced Rotation.
AAV-Syn models have also been shown to display non-motor disturbances. The forced swim test and sucrose preference test, which measure depressive-like behavior and anhedonia, respectively, are altered following bilateral AAV-Syn injections. It is also possible to use AAV-Syn injections to model gastrointestinal (GI) dysfunction.
What are the neuropathologic changes in these models?
AAV-Syn expression leads to the progressive loss of dopaminergic neurons in the substantia nigra pars compacta beginning at approximately 3-4 weeks post-injection. Preceding neurodegeneration is the rapid, early loss of striatal DA terminals and dopamine transporter dysfunction. This progressive time course of degeneration and DA dysfunction along the nigrostriatal axis closely replicates the patterns seen in human PD.
Apart from the abundant increase in transduced α-synuclein at the target site, Oliveras-Salva et al. and Bourdenx et al. showed that AAV-Syn can also lead to increased phosphorylated α-synuclein (pSer129). Daher and colleagues (Daher, 2014; Daher, 2015) identified nitrosylated α-synuclein, while Azaredo de Silviera et al. and Oliveras-Salva et al. showed increased proteinase K and urea-resistant α-synuclein aggregates in response to AAV-Syn injections.
AAV-Syn also induces a robust neuroinflammatory phenotype. Inflammatory changes include increased microglial activation, pro-inflammatory cytokine expression, innate immunity, T-cell infiltration, inflammasome expression, and astrocytosis (Theordore, 2008; Karikari, 2022; Grotemeyer, 2023).
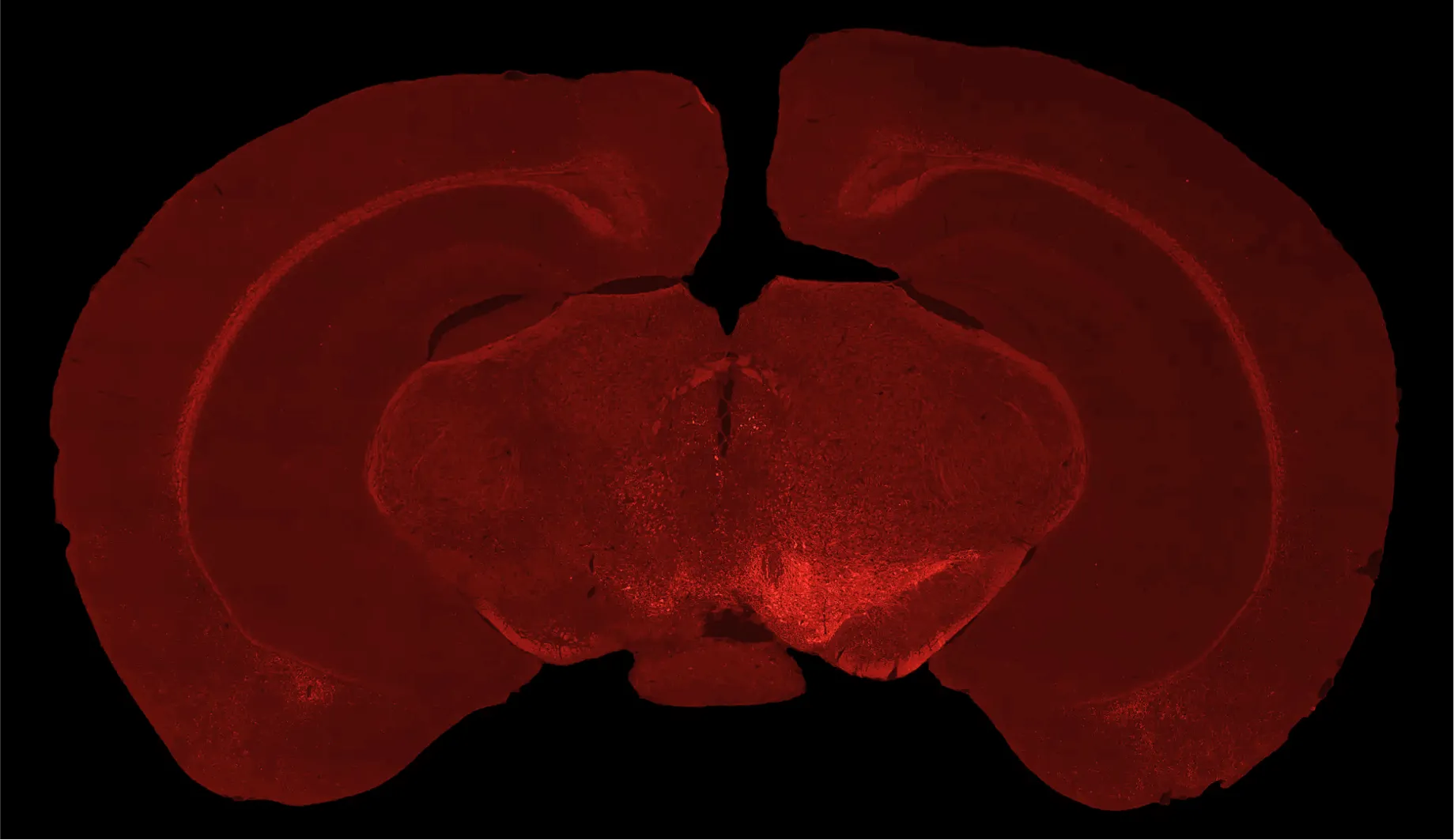
Tyrosine hydroxylase immunofluorescence staining demonstrating dopaminergic neuron loss in the ipsilateral substantia nigra (left hemisphere) of C57BL/6 mice injected with AAV-hA53Tα-Syn.
Our team would be happy to answer any questions about the AAV α-synuclein mouse model or provide specific information about the Parkinson's disease animal models that we use for therapeutic efficacy studies.
Discover more about our Parkinson's Disease Models
Related Content
Up-to-date information on Parkinson's Disease and best practices related to the evaluation of therapeutic agents in PD animal models.
Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease
How α-synuclein influences microglia and astrocytes in Parkinson’s disease and other synucleinopathies.
Autophagy, Parkinson's Disease, and Dopaminergic Neurons
An overview of how impaired autophagy can lead to pathologic changes and neurodegeneration in dopaminergic neurons in Parkinson’s disease.
Neurofilament Light Chain in Parkinson's Disease Models
How neurofilament light chain (NfL; NF-L) levels can be used as blood (plasma; serum) & CSF biomarkers in Parkinson's disease mouse and rat models.
Microglial Activation in an α-Synuclein PFF Mouse Model
We have quantified microglial activation, based on morphology, in an α-synuclein preformed fibril (PFF) seeding & spreading mouse model of Parkinson’s disease.
Brain Atrophy Analysis in Mouse Models of Neurodegeneration
Automated in vivo MRI-based quantitative brain atrophy measures (regional brain volumes and cortical thickness) from mouse models of ALS & Parkinson's disease.