What is the role of NF-κB?
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is defined as a group of proteins that function as transcription factors, playing a crucial role in regulating the expression of genes associated with various cellular functions, including:
- Innate and adaptive immune response and inflammation, including the regulation of inflammasomes (Oeckinghaus, 2009; Liu, 2017).
- Cell proliferation, cell survival (anti-apoptosis), metabolism, cellular stress responses, and synaptic plasticity (Oeckinghaus, 2009; Albensi, 2019; Capece, 2022).
The NF-κB transcription factor family includes NF-κB1 (p105/p50), NF-κB2 (p100/p52), RELA (p65), RELB, and c-REL (Barnabei, 2021; Capece, 2022; Guo, 2024). NF-κB plays varying roles depending on the type of immune cell involved (Zinatizadeh, 2021; Anilkumar, 2024; Guo, 2024):
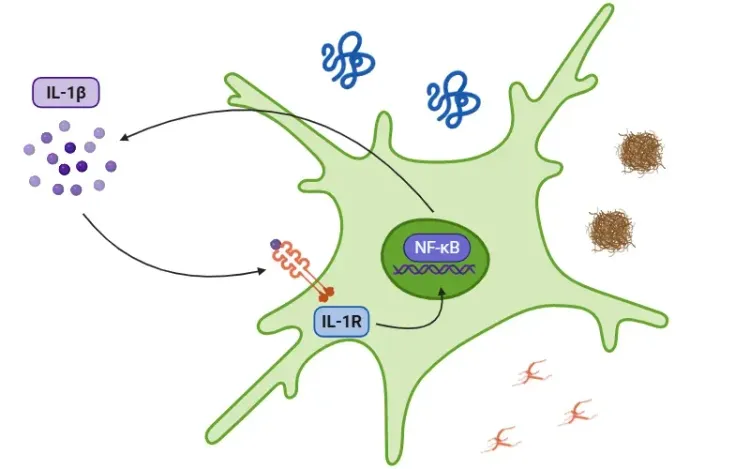
- Macrophages and dendritic cells: NF-κB promotes inflammation through the release of cytokines such as TNFα, IL-1β, IL-6, IL-12, and COX-2.
- For more information on cytokines in neuroinflammation, refer to our Resources: "What is IL-1β?", "IL-1β and Neurodegenerative Diseases", "TNF-α & Microglia in Neurodegenerative Diseases", and "TNF-α & Astrocytes in Neurodegenerative Diseases".
- T cells: NF-κB stimulates cytokine production, primarily IL-2, which is essential for the proliferation and differentiation of T cells into subtypes such as Th1, Th2, Th17, and Treg.
- B cells: NF-κB facilitates the proliferation, survival, and differentiation of B cells.
NF-κB signalling pathway
- In general, NF-κB is bound to an inhibitory protein called IκB (which includes p100, p105, IκBα, and IκBβ) to keep it inactive in the cytoplasm.
- The I-kappaB kinase (IKK) family activates NF-κB, which then translocates to the nucleus, where it promotes gene expression.
There are two major signalling pathways:
- Canonical pathway (Oeckinghaus, 2009; Liu, 2017; Capece, 2022):
- Activation occurs through pro-inflammatory signals, including cytokines (e.g. TNFα, IL-1β), bacterial and viral products, reactive oxygen species (ROS), and radiation, among others. Key receptors involved include interleukin-1 receptor (IL-1R), toll-like receptors (TLR), TNF receptors (TNFR), T-cell receptors (TCR), and B-cell receptors (BCR).
- Each signal has a distinct mechanism for activating the IKK complex through different tumor necrosis factor receptor-associated factors (TRAF).
- Overall, the cascade activates transforming growth factor-β-activated kinase 1 (TAK1), which in turn activates the IKK complex (IKKα and IKKβ) and its related kinase, NEMO (also known as IKKγ).
- Activated IKK phosphorylates IκB (mainly IκBα) and allows NF-κB translocation into the nucleus.
- In the nucleus, NF-κB dimers bind to κB DNA sequences that promote gene transcription for:
- Pro-inflammatory cytokines such as IL-1β, IL-6, and TNFα, to further amplify the inflammatory response.
- Chemokines to attract other immune cells.
- Adhesion molecules to attract leukocytes to the inflamed tissue.
- Other inflammatory mediators such as COX-2 and iNOS.
- Non-canonical pathway (Lawrence, 2009; Oeckinghaus, 2009; Liu, 2017):
- The activation of this pathway is slower than the canonical pathway.
- Specific receptors from the TNF superfamily trigger this pathway, including B lymphocyte activating factor receptor (BAFF-R), CD40, lymphotoxin β receptor (LTβR), and receptor activator of NF-κB (RANK).
- Activation of these receptors recruits different types of TRAF members to activate NF-κB inducing kinase (NIK).
- Subsequent activation of NIK leads to phosphorylation of IKKα homodimers.
- NIK and IKKα phosphorylate p100 (NF-κB2 precursor protein), converting it into the active form known as p52.
- P52 form the p52/RelB dimer and translocates to the nucleus to promote gene transcription, regulating immune cell survival, communication, development, and maturation of T and B cells.
Since many signalling molecules are activated in both canonical and non-canonical pathways, there is direct and indirect crosstalk with other important signalling pathways, such as PI3K/AKT, MAPK, JAK-STAT, and TGF-β (Guo, 2024).
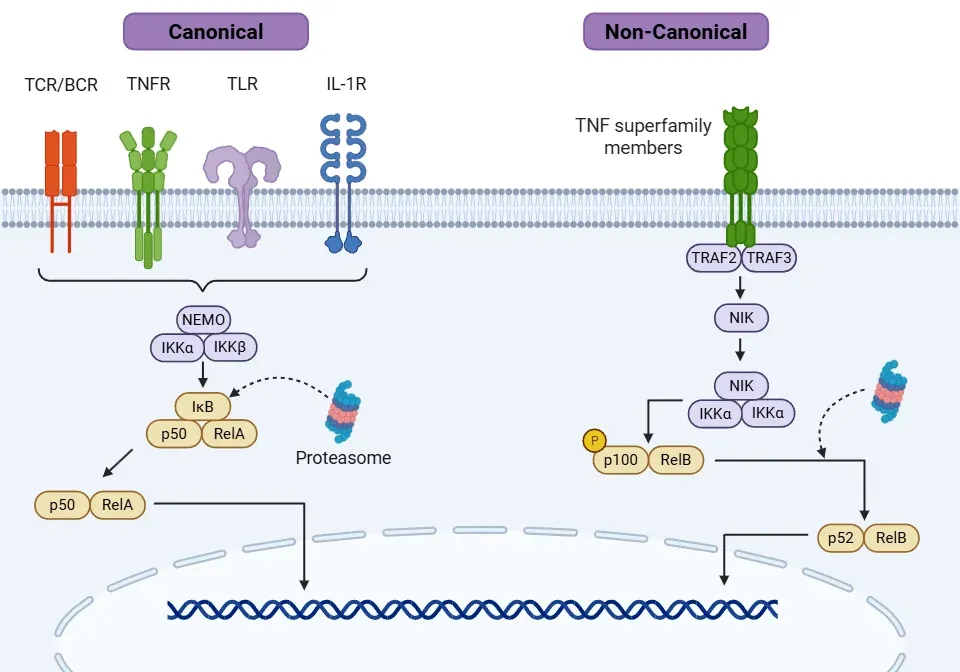
NF-κB signalling through the canonical and non-canonical pathways.
What are the implications of NF-κB dysregulation in disease?
The role of NF-κB is complex and context-dependent, and it can be considered beneficial or detrimental (Albensi, 2019). While NF-κB is essential for homeostasis, problems in regulating NF-κB are associated with various diseases, including neurological diseases, cancer, inflammatory, and autoimmune diseases.
Neurological Diseases
Under physiological conditions, NF-κB activity in neurons is necessary for normal neuronal function, including neurogenesis, neuritogenesis, and synaptic plasticity (Shih, 2015).
- In neurons, NF-κB typically promotes survival by inducing anti-apoptotic genes, such as Bcl-2, Bcl-XL, and manganese superoxide dismutase (Anilkumar, 2024).
- In microglia, NF-κB activation drives pro-inflammatory responses, leading to the release of cytokines (TNF-α, IL-1β, IL-6, IFN-γ) and ROS (Shih, 2015; Anilkumar, 2024).
- Refer to our Resource “TNF-α & Microglia in Neurodegenerative Diseases”.
- In astrocytes, NF-κB activation is necessary for astrocytic differentiation and increases the inflammatory response (Anilkumar, 2024).
- Refer to our Resource “TNF-α & Astrocytes in Neurodegenerative Diseases”.
However, dysregulated activation of NF-κB contributes to pathological conditions:
- Multiple Sclerosis (MS):
- NF-κB mediates neuroinflammation through infiltrating macrophages, microglia, astrocytes, and peripheral immune cells.
- NF-κB is also necessary for the transition of T cells into Treg, promoting the pathogenic actions of inflammatory T cells (Guo, 2024).
- In experimental autoimmune encephalomyelitis (EAE), NF-κB activation triggers neuroinflammation. Interestingly, deleting IKKβ specifically in myeloid cells prevents the induction of EAE, which is associated with a decrease in the production of inflammatory Th1 and Th17 cells (Liu, 2017).
- Alzheimer’s disease (AD) & Parkinson’s disease (PD):
- In these diseases, microglial activation of NF-κB promotes further release of pro-inflammatory cytokines via reactive microglia and astrocytes, leading to neurotoxicity and contributing to neurodegeneration (Guo, 2024).
- However, in AD mouse models, inhibiting NF-κB has been shown to accelerate the accumulation of amyloid-beta (Aβ) and tau, suggesting that NF-κB may also play a protective role in the early stages of the disease (Jong Huat, 2024).
- Refer to our Resources: “APOE4, Microglia & Alzheimer’s Disease”, “Microglia, Astrocytes & Tau in Neurodegenerative Disorders”, and “Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease”.
- Traumatic brain injury and spinal cord injury:
- NF-κB plays a role in the inflammatory response after the acute event, thereby contributing to secondary injury (Guo, 2024).
Cancer
- NF-κB may promote tumorigenesis primarily through the abnormal activation of the non-canonical pathway. The mechanisms differ for each type of cancer. In general, the mechanisms include the following (Aggarwal, 2011; Xia, 2014):
- Promotion of cell survival and proliferation: NF-κB contributes to the uncontrolled division of cancer cells by enhancing the expression of cyclins D1 and E, as well as c-Myc, while inhibiting apoptosis through factors such as Bcl-2, Bcl-XL, and IAPs (Brown, 2008; Rinkenbaugh, 2016).
- For more information about pyroptosis, apoptosis, and necroptosis, refer to the Resource “What is Pyroptosis?”.
- Promotion of angiogenesis: NF-κB facilitates the formation of new blood vessels through pro-angiogenic factors like VEGF, FGF, PDGF, and IL-8, as well as triggering epithelial-mesenchymal transition, which supports tumor growth and metastasis (Brown, 2008; Park, 2016).
- Immune evasion: NF-κB contributes to the development of an immunosuppressive tumor microenvironment via TGF-β (Rinkenbaugh, 2016; Guo, 2024).
- Metabolic reprogramming: NF-κB alters metabolic pathways, leading to increased demand for nitrogen and abnormal mitochondrial oxidative phosphorylation (Guo, 2024).
- Promotion of cell survival and proliferation: NF-κB contributes to the uncontrolled division of cancer cells by enhancing the expression of cyclins D1 and E, as well as c-Myc, while inhibiting apoptosis through factors such as Bcl-2, Bcl-XL, and IAPs (Brown, 2008; Rinkenbaugh, 2016).
- Direct mutations activating NF-κB are common in blood cancers. For instance, c-Rel amplification occurs in non-Hodgkin lymphomas of B-cell origin, and NF-κB2/p100 is frequently activated through chromosomal translocations (Xia, 2014).
- Abnormal NF-κB regulation has been identified in various cancer types, including squamous cell carcinomas, breast cancer, lung cancer, and colorectal cancer (Brown, 2008).
Autoimmune and Inflammatory Diseases
- Rheumatoid arthritis (RA): NF-κB perpetuates chronic inflammation and has been found in the synovial fluid of patients with RA. It promotes the production of inflammatory cytokines (IFNγ, IL-15, IL-17, IL-18) in T cells in the joints and the differentiation of bone-resorbing osteoclasts (Liu, 2017; Guo, 2024).
- Inflammatory bowel disease (IBD): This condition is associated with genetic mutations in NF-κB, which have been identified in diseases such as Crohn’s disease and ulcerative colitis. In patients with IBD, the activation of NF-κB has been observed in their colonic tissues (Liu, 2017).
- Other autoimmune diseases associated with dysregulation of NF-κB include systemic lupus erythematosus and type 1 diabetes (Guo, 2024).
- Inflammatory diseases with dysregulation of NF-κB include gout, asthma, acute kidney injury, atherosclerosis, myocardial infarction, and COVID-19 infection (Lawrence, 2009; Liu, 2017).
NEMO Deficiency Syndrome
- NEMO deficiency syndrome is an X-linked genetic disorder characterized mainly by ectodermal dysplasia and immune deficiency, which increases susceptibility to infections.
- This condition results from a mutation in the IKBKG gene, which encodes the NEMO protein. The mutation leads to the increased activation of NF-κB (Pescatore, 2022).
What potential therapeutic strategies target NF-κB?
A variety of therapeutic strategies have been developed to target the NF-κB signalling pathway at different points. The dual role of NF-κB, being both anti-tumorigenic and pro-tumorigenic, complicates its therapeutic applications (Xia, 2018).
- IKK inhibitors (Yu, 2020; Guo, 2024; Li, 2024):
- Aspirin: An inhibitor of COX and IKKβ, used in animal models for various cancers.
- Sulfasalazine: An antibiotic that inhibits the expression of TLR4, MyD88, and NF-κB p65 proteins, used in models in vivo to prevent bone metastasis.
- Dexamethasone: A glucocorticoid that inhibits the RelA subunit of NF-κB.
- Antitumor drugs: Thalidomide and Lenalidomide inhibit NF-κB activation and show potential in the treatment of lung cancer, leukemia, and multiple myeloma.
- Monoclonal antibodies (Yu, 2020; Guo, 2024):
- Anti-PD1/PD-L1: Includes pembrolizumab, atezolizumab, avelumab, durvalumab, and nivolumab.
- Anti-β2-microglobulin monoclonal antibodies: Aim to reduce NF-κB activity and are under investigation for multiple myeloma.
- Anti-IL1: Anakinra, Rilonacept, and Canakinumab inhibit the activation of NF-κB via the IL-1R in autoimmune diseases, such as CAPS.
- Anti-TNFα: Adalimumab, Infliximab, and Etanercept inhibit the activation of NF-κB and are used in different types of cancer.
- Proteasome inhibitors (Rasmi, 2020; Guo, 2024; Li, 2024):
- Bortezomib, Carfilzomib, Ixazomib: Inhibit IκBα degradation and suppress NF-κB activity. They are FDA-approved for the treatment of multiple myeloma, diffuse large B-cell lymphoma, and non-small cell lung cancer.
- Bortezomib, Carfilzomib, Ixazomib: Inhibit IκBα degradation and suppress NF-κB activity. They are FDA-approved for the treatment of multiple myeloma, diffuse large B-cell lymphoma, and non-small cell lung cancer.
- Inhibitors of nuclear translocation (Sivamaruthi, 2023; Guo, 2024):
- Tacrolimus: An immunosuppressive agent. it blocks the activation of the nuclear factor of activated T cells, thereby inhibiting the activation of NF-κB.
- IκBα super-repressor: Continuous suppression of NF-κB.
- SN50 peptide inhibits NF-κB by competing with the protein complexes responsible for nuclear translocation.
- A nanoligomer targeting NF-κB and NLRP3 inflammasome, inhibiting its transcription and translation. This resulted in decreased neuroinflammation and improved cognitive function in AD mouse models (Wahl, 2024).
- Refer to our Resource “NLRP3 Inflammasome and Neurodegenerative Diseases”.
Some medications not explicitly developed as NF-κB inhibitors have been found to interfere with NF-κB activation (Roberti, 2022):
In MS:
- Imatinib mesylate: Inhibits various tyrosine kinases and also inhibits IκB phosphorylation, currently undergoing Phase 2 clinical trials for MS (Barnthaler, 2019).
- Topotecan: Inhibits IKKβ, reducing inflammation in EAE mouse models (Roberti, 2022).
In Alzheimer’s disease:
- Alogliptin: A hypoglycemic medication that can modulate the TLR4/MyD88/NF-κB pathway in vitro (El-Sahar, 2021).
- Telmisartan: A medication for high blood pressure that may indirectly reduce NF-κB activity via IL-1β reduction (Sivamaruthi, 2023).
In Stroke:
- Modafinil: Inhibits NF-κB and provides anti-inflammatory effects, reducing neuronal degeneration in the ischemic hippocampus (Xu, 2024).
- Atorvastatin: Ameliorates neurological deficits by inhibiting HMGB1-induced NF-κB activation (Xu, 2024).
Targeting the NF-κB signalling pathway thus represents a promising approach for developing therapeutic strategies for various diseases, including neurological diseases, cancer, and autoimmune conditions. A comprehensive understanding of NF-κB's roles is essential for optimizing these strategies and improving treatment outcomes across various disease contexts.
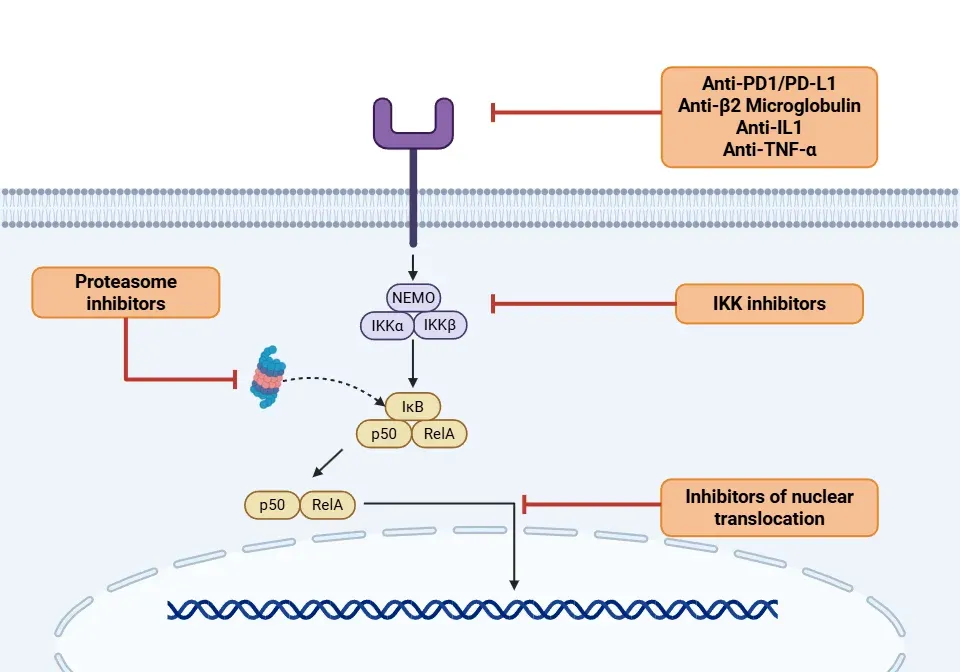
The mechanism of action of NF-κB therapies illustrated through its signalling pathway.
Our team would be happy to answer any questions about Nuclear Factor Kappa B or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
What Is IL-1β (IL-1b)? Function, Signaling, and Biological Role
An overview of IL-1β, including its signaling pathways, involvement in disease mechanisms, and potential therapeutic targets.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
TNF-α (TNF-alpha) & Microglia in Neurodegenerative Diseases
An overview of the function of tumor necrosis factor-alpha (TNF-α) in microglia and its contribution to the progression of neurodegeneration.
TNF-α & (TNF-alpha) Astrocytes in Neurodegenerative Diseases
An overview of TNF-α signaling in astrocytes, its role in neurodegeneration, and therapeutic strategies targeting this pathway..
Inflammasome – A Therapeutic Target for Multiple Diseases
An overview of inflammasomes, including their mechanisms of action, roles in diseases, and targeting for drug development.
What is Pyroptosis? | A Drug Development Perspective
An overview of pyroptosis, its role in various diseases, and therapeutic strategies related to pyroptosis pathways.