What is pyroptosis?
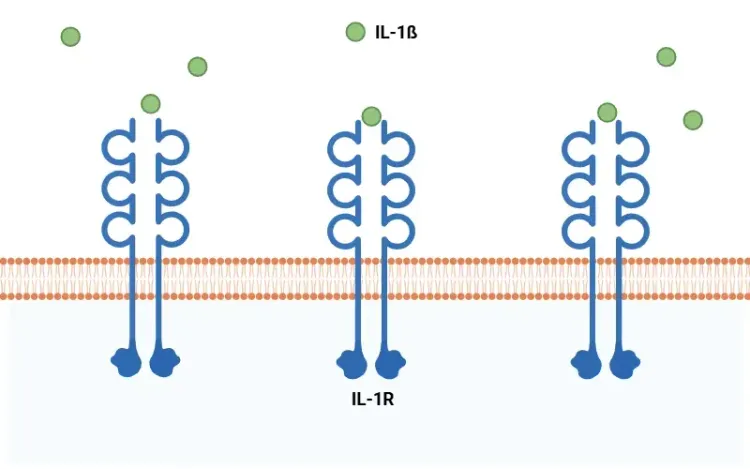
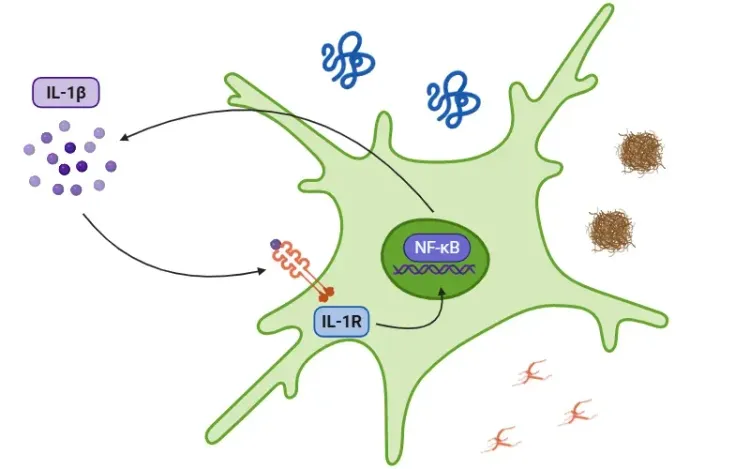
Pyroptosis is a pro-inflammatory form of programmed cell death characterized by cell swelling, the formation of pores in the plasma membrane, membrane rupture, and the subsequent release of pro-inflammatory intracellular contents such as IL-1β and IL-18 (Vande Walle, 2016; Fang, 2020; Tan, 2021; Rao, 2022).
See: What is IL-1β (Interleukin-1 Beta)? & Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
This process is part of the innate immune system, serving as a primary defense mechanism against pathogens by expelling them into the extracellular space, where they can be targeted by the immune response (Vande Walle, 2016). However, when activated excessively, pyroptosis can lead to widespread cell death, tissue damage, and an inflammatory response that can contribute to various inflammatory and autoimmune diseases (Shi, 2017; Rao, 2022; Song, 2022).
The term "pyroptosis" was first introduced in 2001 and originates from the Greek words "pyro", which means fire or fever, and "ptosis", meaning falling. This terminology reflects the pro-inflammatory nature of this cell death pathway. Notably, pyroptosis differs from other forms of cell death, such as apoptosis and necroptosis (Fang, 2020; Y. Liu, 2024):
- Apoptosis is primarily involved in tissue development and maintaining homeostasis, while also helping to eliminate aging or slightly damaged cells. It ensures a clean process by removing apoptotic bodies, thereby preventing the triggering of an inflammatory response (Bertheloot, 2021).
- Necroptosis, similar to pyroptosis, also initiates an inflammatory response, but it is executed by different molecules (specifically RIPK1, RIPK3, and MLKL) and serves as a backup mechanism against pathogens that have managed to inhibit apoptosis (Bertheloot, 2021).
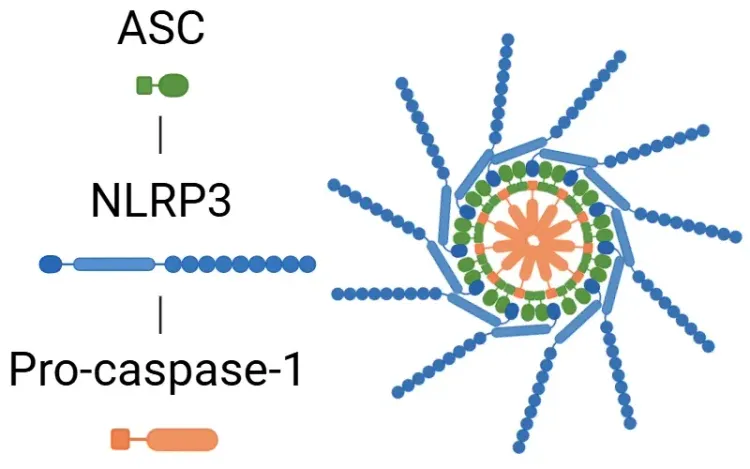
Pyroptosis is generally triggered by the activation of various caspases, which are enzymes that cleave gasdermins (GSDM: A, B, C, D, E, and DFNB59 or PVJK). Among these, Gasdermin D has been the focus of extensive research due to its presence in a wide range of immune cells, organs, and tissues. When GSDM is cleaved, it splits into an N-terminal fragment and a C-terminal fragment. The N-terminal fragment then moves to the plasma membrane, where it oligomerizes to create large pores. These pores lead to the influx of water and an imbalance of ions, ultimately causing cell swelling and resulting in osmotic lysis (Bergsbaken, 2009; Fang, 2020; Yu, 2021).
There are four main signaling pathways involved in the process of pyroptosis (Yu, 2021):
- Canonical Inflammasome Pathway
- Activation: This pathway is triggered by Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs), leading to the activation of the inflammasome components, including NLRP1, NLRP3, NLRC4, and AIM2.
- Following activation, the inflammasome activates caspase-1.
- Caspase-1 then cleaves GSDMD, initiating pyroptosis, and cleaves pro-IL-1β and pro-IL-18 into their active forms (Bertheloot, 2021; Tan, 2021; Song, 2022).
- See: What is an Inflammasome & What is NLRP3?
- Non-Canonical Pathway
- Activation: This pathway is primarily initiated by cytoplasmic lipopolysaccharides (LPS) from bacteria, which activate specific macromolecular signal complexes (caspases 4/5 in humans and caspase 11 in mice).
- Caspases 4/5/11 cleave GSDMD, leading to pyroptosis independently of caspase-1.
- Cell membrane pores are formed by GSDMD and P2X7.
- Efflux of potassium ions further contributes to assembling the NLRP3 inflammasome (Fang, 2020; Yu, 2021; Song, 2022).
- Caspase 3/8 Pathway
- This pathway involves caspases traditionally linked to apoptosis, specifically caspase-3 and caspase-8.
- Caspase 3: Its activation can result from chemotherapy and immunotherapy (g. CAR T cells), ultimately cleaving GSDME.
- Caspase 8: Activation occurs in response to specific infections, such as those caused by Yersinia or Aspergillus, resulting in the cleavage of GSDMD. There is also an alternative pathway, activated by TNF-α under hypoxic conditions in cancer cells, which may activate GSDMC (Yu, 2021; Chai, 2023).
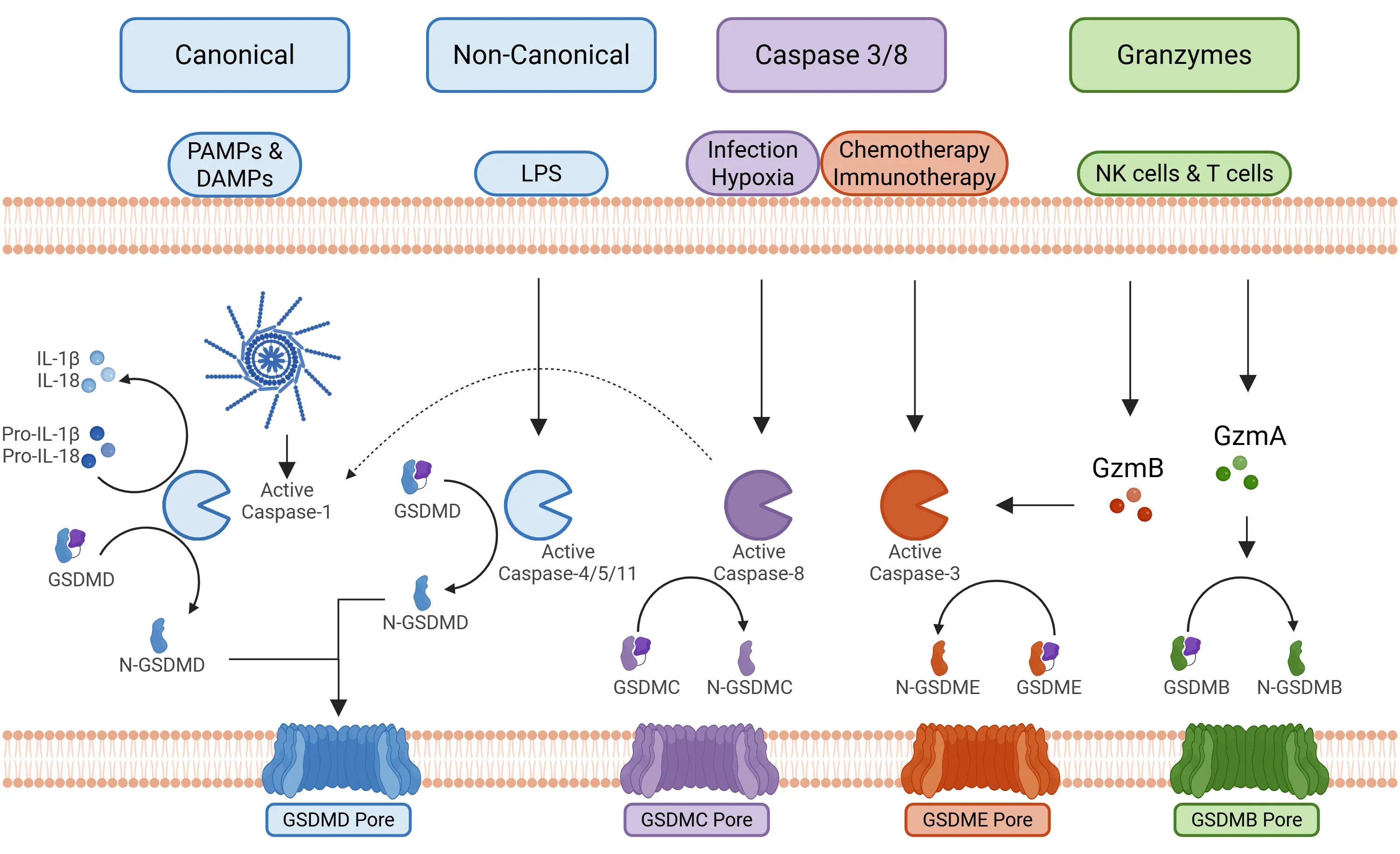
Primary signaling pathways promoting pyroptosis.
Is pyroptosis implicated in diseases?
Pyroptosis contributes to neuroinflammation and neuronal damage by causing cells to rupture and release pro-inflammatory molecules, creating a detrimental environment within the central nervous system (CNS). This process affects various cell types within the brain, each contributing to disease pathology in distinct ways:
- Microglia are the central immune cells of the CNS and play a key role in neuroinflammation. When they undergo pyroptosis, they enter a pro-inflammatory state that releases inflammatory mediators, which can damage neurons and impair their ability to eliminate harmful substances, such as misfolded proteins that contribute to disease progression (Wu, 2022; Oladapo, 2024).
- Astrocytic pyroptosis leads to cell swelling, the formation of membrane pores, and the release of neurotoxins. This process disrupts the supportive roles of astrocytes, leading to mitochondrial dysfunction and endoplasmic reticulum stress, which in turn exacerbates the neuroinflammatory environment (Oladapo, 2024).
- Neurons can also undergo pyroptosis, directly contributing to the neuronal loss observed in neurodegenerative diseases, strokes, and infections (Wu, 2022).
Neurological Diseases
Specific involvement of pyroptosis in neurological diseases:
- Alzheimer’s disease (AD):
- In AD, aggregates of amyloid-beta and neurofibrillary tangles trigger microglia to activate the NLRP3 inflammasome, initiating pyroptosis via GSDMD. This process releases inflammatory cytokines that promote neuroinflammation and reduce the ability of microglia to clear Aβ plaques, perpetuating a vicious cycle of pathology (Wu, 2022; Y. Liu, 2024; Oladapo, 2024).
- For more information on neuroinflammation in AD, refer to our Resource: Microglia, Astrocytes & Tau in Neurodegenerative Diseases.
- Parkinson’s disease (PD):
- Misfolded α-synuclein protein can activate the NLRP3 inflammasome in both microglia and neurons. This activation results in the cleavage of GSDMD, which forms pores in cell membranes, leading to pyroptosis. Consequently, pro-inflammatory cytokines are released, perpetuating neuroinflammation and progressive loss of dopaminergic neurons (Wu, 2022; Liang, 2024; Oladapo, 2024).
- For more information on neuroinflammation in PD, refer to our Resource: Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease.
- Multiple sclerosis (MS):
- Evidence indicates that pyroptosis occurs in both microglia and oligodendrocytes within MS lesions, facilitated by caspase-1 and GSDMD. This contributes to inflammation and demyelination (Y. Liu, 2024).
- Ischemic stroke:
- Pyroptosis in the CNS is a major driver of secondary injury after a stroke. Methyltransferase-like 14 (METTL14) activates the NLRP3 inflammasome, promoting pyroptosis. The resulting neuroinflammation can exacerbate the initial damage and has been linked to a delayed recovery (Wu, 2022; Y. Liu, 2024).
Cancer
Pyroptosis plays a complex, dual role in cancer, acting as both a tumour promoter and suppressor depending on the specific cancer type and molecular context. For instance:
- In melanoma, NLRP1 and NLRP3 inflammasomes can promote tumor growth and create an immunosuppressive environment by activating myeloid-derived suppressor cells (MDSC). Conversely, inducing GSDME-dependent pyroptosis through chemotherapy can inhibit tumor growth and metastasis (Huang, 2022; Z. Liu, 2024).
- In breast cancer, overexpression of GSDMB and GSDMC is linked to tumor growth, metastasis, and poor prognosis. However, reduced GSDMD-regulated pyroptosis is associated with disease progression (Huang, 2022; Z. Liu, 2024).
- In colorectal cancer, deficiencies in AIM2 and caspase-1 are associated with poor prognoses, indicating a tumor-suppressive role. However, NLRP3 activation might promote metastasis, and high GSDMD levels are correlated with a poor prognosis (Huang, 2022; Z. Liu, 2024).
Inflammatory Diseases
Due to the role of pyroptosis in driving excessive cytokine release and inflammation, it is involved in inflammatory diseases, including:
- Autoinflammatory diseases (Vande Walle, 2016; Song, 2022; Chai, 2023):
- Cryopyrin-associated periodic syndrome (CAPS)
- Familial Mediterranean fever (FMF)
- Pyrin-associated auto-inflammation with neutrophilic dermatosis (PAAND)
- Autoinflammation with infantile enterocolitis (AIFEC)
- Sterile inflammatory diseases (Wu, 2022; Y. Liu, 2024):
- Cardiovascular disease: Atherosclerosis, myocardial infarction
- Liver disease: Non-alcoholic fatty liver, non-alcoholic steatohepatitis (NASH), and liver fibrosis
- Lung disease: Asthma and silicosis
Are there possible therapeutic applications that target pyroptosis?
Targeting pyroptosis presents a promising therapeutic strategy for a wide range of diseases, including cancers, inflammatory disorders, and neurological conditions. The therapeutic approach depends on the disease context; inducing pyroptosis is beneficial for treating cancer, while inhibiting it is the goal for inflammatory and autoimmune diseases.
Gasdermin-Related Therapies:
- Inhibition:
- These drugs can reduce pathological inflammation by preventing oligomerization of GSDMD-N-terminal and pore formation (Y. Liu, 2024; Oladapo, 2024; Zhu, 2024):
- Dimethyl Fumarate: Approved as a therapeutic agent for MS. It has been used in several animal models to reduce the severity of experimental autoimmune encephalomyelitis (EAE) and FMF.
- C202-2729: This compound has been used in animal models to inhibit inflammation in EAE and endotoxin shock.
- Disulfiram and Necrosulfonamide: These drugs have been used in various animal models to treat various inflammatory diseases, such as Alzheimer’s disease, sepsis, COVID-19, and atherosclerosis, among others.
- These drugs can reduce pathological inflammation by preventing oligomerization of GSDMD-N-terminal and pore formation (Y. Liu, 2024; Oladapo, 2024; Zhu, 2024):
-
- Caspase-1 inhibitors can prevent the cleavage of GSDMD and the subsequent pyroptosis (Wu, 2022; Oladapo, 2024):
- VX-765 has been shown to reduce neuroinflammation in animal models of EAE.
- Ac-YVAD-CMK has also been used in preclinical studies.
- Caspase-1 inhibitors can prevent the cleavage of GSDMD and the subsequent pyroptosis (Wu, 2022; Oladapo, 2024):
-
- LDC7559 treatment has been shown to reduce neuronal apoptosis and neuroinflammation, ultimately improving behavioural and functional recovery after subarachnoid hemorrhage in research models (Cai, 2023).
- Activation: To promote antitumor immunity (see the table below for specific examples):
- GSDME agonists: Chemotherapy drugs such as cisplatin, doxorubicin, and paclitaxel can activate caspase-3, cleave GSDME, and trigger pyroptosis in different types of cancer cells, including breast and lung cancer (Fu, 2020; Liao, 2022; Magnani, 2022; Privitera, 2023; Y. Liu, 2024).
- GSDMD agonists: Drugs like sorafenib and cisplatin can activate caspase-1 and cleave GSDMD to trigger pyroptosis in specific cancers, including hepatocellular carcinoma and triple-negative breast cancer (Liao, 2022; Y. Liu, 2024).
- GSDMA3 conjugated to nanoparticles creates an anti-tumour effect in the presence of cytotoxic T cells and T helper cells (Yu, 2021).
Inflammasome Inhibitors: The NLRP3 inflammasome is an upstream activator of pyroptosis and another focus for drug development:
- MCC950 derivatives, for instance, are being used for neurodegenerative diseases in animal models (Wu, 2022; Y. Liu, 2024). For more information on therapeutic targets of the inflammasome, please refer to our resources: NLRP3 Inflammasome and Neurodegenerative Diseases & What is an inflammasome?
While these therapies are promising for various inflammatory diseases, including neurodegenerative conditions, there are challenges associated with gasdermin-related therapies. As noted earlier, the functions of gasdermins are context-dependent; they may promote inflammation in macrophages while supporting homeostasis in intestinal mucosal cells. Consequently, the use of gasdermin-related therapies carries a risk of toxicity to healthy cells through hyper-pyroptosis, potentially leading to systemic consequences due to a lack of specificity.
| Target | Drug or Compound | Cancer Type |
| GSDME/Caspase-3 | Paclitaxel & Cisplatin |
Lung Cancer |
| Loboplatin |
Cervical cancer | |
| Doxorubicin |
Breast cancer | |
| Dihydroartemisinin (DHA) |
Breast cancer | |
| Polo-like kinase 1 (PLK1) inhibitor |
Esophageal squamous cell carcinoma | |
| Tetraarsenic hexoxide |
Triple-negative breast cancer | |
GSDMD/Caspase-1 | Sorafenib |
Hepatocellular carcinoma |
| Cisplatin |
Triple-negative breast cancer | |
| FL118 |
Colorectal cancer | |
| Alpine pine flavones (AIF) |
Hepatocellular carcinoma | |
| Metformin |
Hepatocellular carcinoma | |
| VB12-sericin-PBLG-IR780 nanoparticle |
Gastric cancer | |
| Nanoparticle with a calcium chelator |
Breast cancer, melanoma, ovarian cancer |
Our team would be happy to answer any questions about pyroptosis or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Inflammasome – A Therapeutic Target for Multiple Diseases
An overview of inflammasomes, including their mechanisms of action, roles in diseases, and targeting for drug development.
What is NLRP3?
An overview of NLRP3 inflammasome activation triggers, disease associations, and therapeutic targeting strategies.
What Is IL-1β (IL-1b)? Function, Signaling, and Biological Role
An overview of IL-1β, including its signaling pathways, involvement in disease mechanisms, and potential therapeutic targets.
Interleukin-1 Beta (IL-1β) and Neurodegenerative Diseases
The role of IL-1beta in neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS).
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.