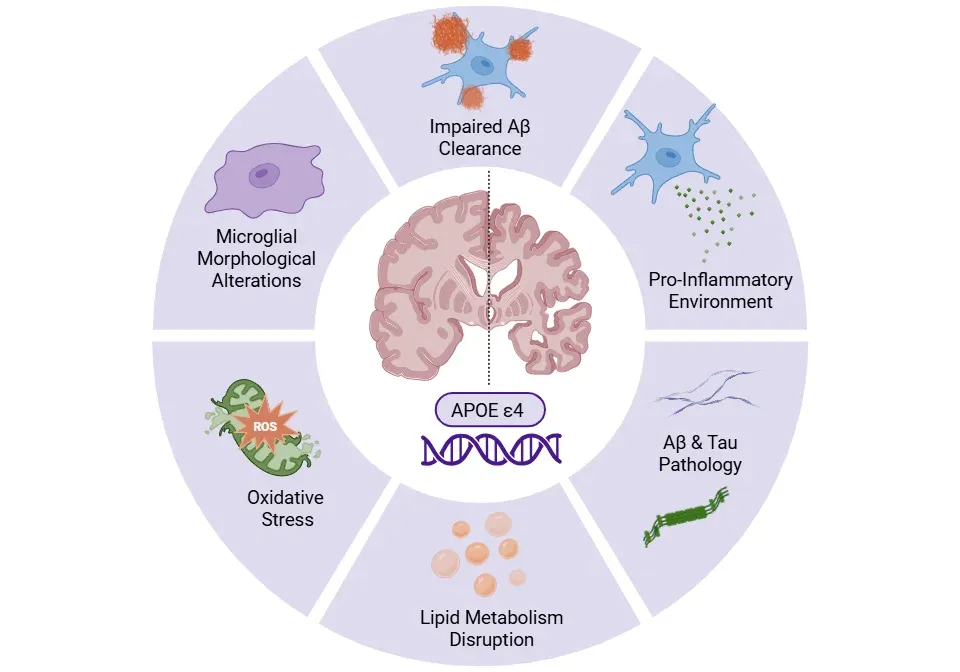
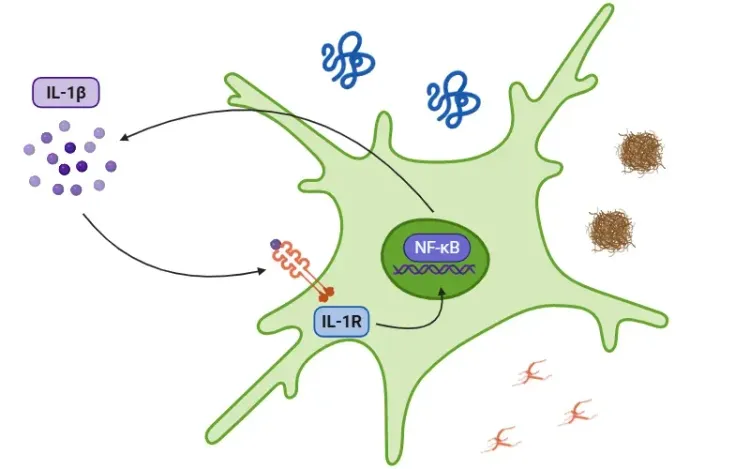
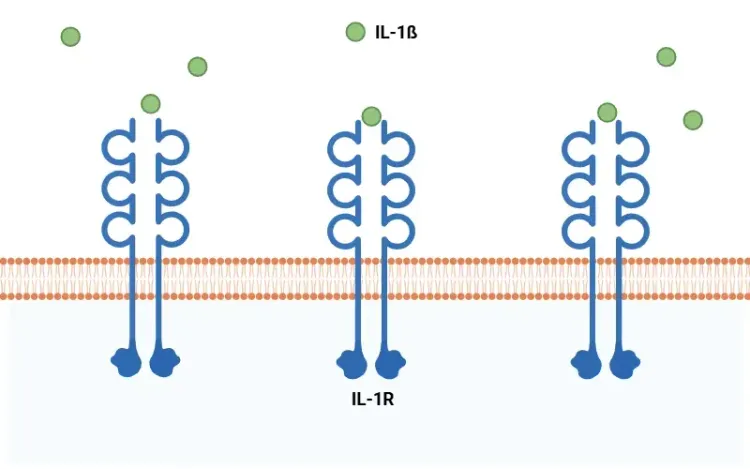
Blanchard, J.W., Akay, L.A., Davila-Velderrain, J., von Maydell, D., Mathys, H., Davidson, S.M., Effenberger, A., Chen, C.Y., Maner-Smith, K., Hajjar, I., Ortlund, E.A., Bula, M., Agbas, E., Ng, A., Jiang, X., Kahn, M., Blanco-Duque, C., Lavoie, N., Liu, L., Reyes, R., Lin, Y.T., Ko, T., R'Bibo, L., Ralvenius, W.T., Bennett, D.A., Cam, H.P., Kellis, M. ,Tsai, L. H. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature, 611:769-779, 2022; doi: 10.1038/s41586-022-05439-w
Davis, A.A., Inman, C.E., Wargel, Z.M., Dube, U., Freeberg, B.M., Galluppi, A., Haines, J.N., Dhavale, D.D., Miller, R., Choudhury, F.A., Sullivan, P.M., Cruchaga, C., Perlmutter, J.S., Ulrich, J.D., Benitez, B.A., Kotzbauer, P.T., Holtzman, D.M. APOE genotype regulates pathology and disease progression in synucleinopathy. Sci. Transl. Med., 12:eaay3069, 2020; doi: 10.1126/scitranslmed.aay3069
Deane, R., Sagare, A., Hamm, K., Parisi, M., Lane, S., Finn, M.B., Holtzman, D.M., Zlokovic, B.V. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Invest., 118:4002-13, 2008; doi: 10.1172/JCI3666
Dias, D., Portugal, C.C., Relvas, J., Socodato, R. From genetics to neuroinflammation: the impact of ApoE4 on microglial function in Alzheimer's disease. Cells, 14:243, 2025; doi: 10.3390/cells14040243
Dickson, D.W., Heckman, M.G., Murray, M.E., Soto, A.I., Walton, R.L., Diehl, N.N., van Gerpen, J.A., Uitti, R.J., Wszolek, Z.K., Ertekin-Taner, N., Knopman, D.S., Petersen, R.C., Graff-Radford, N.R., Boeve, B.F., Bu, G., Ferman, T.J., Ross, O.A. APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology, 91:e1182-e1195, 2018; doi: 10.1212/WNL.0000000000006212
Hashimoto, T., Serrano-Pozo, A., Hori, Y., Adams, K.W., Takeda, S., Banerji, A.O., Mitani, A., Joyner, D., Thyssen, D.H., Bacskai, B.J., Frosch, M.P., Spires-Jones, T.L., Finn, M.B., Holtzman, D.M., Hyman, B.T. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J. Neurosci., 32:15181-92, 2012; doi: 10.1523/JNEUROSCI.1542-12.2012
Huynh, T.V., Liao, F., Francis, C.M., Robinson, G.O., Serrano, J.R., Jiang, H., Roh, J., Finn, M.B., Sullivan, P.M., Esparza, T.J., Stewart, F.R., Mahan, T.E., Ulrich, J.D., Cole, T., Holtzman, D.M. Age-dependent effects of apoE reduction using antisense oligonucleotides in a model of β-amyloidosis. Neuron, 96:1013-1023.e4, 2017; doi: 10.1016/j.neuron.2017.11.014
Iannucci, J., Sen, A., Grammas, P. Isoform-specific effects of apolipoprotein E on markers of inflammation and toxicity in brain glia and neuronal cells in vitro. Curr. Issues Mol. Biol., 43:215-225, 2021; doi: 10.3390/cimb43010018
Imbimbo, B.P., Solfrizzi, V., Panza, F. Are NSAIDs useful to treat Alzheimer's disease or mild cognitive impairment? Front. Aging Neurosci., 2:19, 2010; doi: 10.3389/fnagi.2010.00019
Jendresen, C., Årskog, V., Daws, M.R., Nilsson, L.N. The Alzheimer's disease risk factors apolipoprotein E and TREM2 are linked in a receptor signaling pathway. J. Neuroinflammation, 14:59, 2017; doi: 10.1186/s12974-017-0835-4
Kim, J., Eltorai, A.E., Jiang, H., Liao, F., Verghese, P.B., Kim, J., Stewart, F.R., Basak, J.M., Holtzman, D.M. Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Aβ amyloidosis. J. Exp. Med., 209:2149-56, 2012; doi: 10.1084/jem.20121274
Liao, F., Li, A., Xiong, M., Bien-Ly, N., Jiang, H., Zhang, Y., Finn, M.B., Hoyle, R., Keyser, J., Lefton, K.B., Robinson, G.O., Serrano, J.R., Silverman, A.P., Guo, J.L., Getz, J., Henne, K., Leyns, C.E., Gallardo, G., Ulrich, J.D., Sullivan, P.M., Lerner, E.P., Hudry, E., Sweeney, Z.K., Dennis, M.S., Hyman, B.T., Watts, R.J., Holtzman, D.M. Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation. J. Clin. Invest., 128:2144-2155, 2018; doi: 10.1172/JCI96429
Litvinchuk, A., Huynh, T.V., Shi, Y., Jackson, R.J., Finn, M.B., Manis, M., Francis, C.M., Tran, A.C., Sullivan, P.M., Ulrich, J.D., Hyman, B.T., Cole, T., Holtzman, D.M. Apolipoprotein E4 reduction with antisense oligonucleotides decreases neurodegeneration in a tauopathy model. Ann. Neurol., 89:952-966, 2021; doi: 10.1002/ana.26043
Liu, A., Wang, T., Yang, L., Zhou, Y. The APOE-microglia axis in Alzheimer's disease: functional divergence and therapeutic perspectives-a narrative review. Brain Sci., 15:675, 2025; doi: 10.3390/brainsci15070675
Mahley, R.W., Huang, Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron, 76:871-85, 2012; doi: 10.1016/j.neuron.2012.11.020
McGeer, P.L., McGeer, E.G. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol. Aging, 28:639-47, 2007; doi: 10.1016/j.neurobiolaging.2006.03.013
Mishra, A., Ferrari, R., Heutink, P., Hardy, J., Pijnenburg, Y., Posthuma, D.; International FTD-Genomics Consortium. Gene-based association studies report genetic links for clinical subtypes of frontotemporal dementia. Brain, 140:1437-1446, 2017; doi: 10.1093/brain/awx066
Najm, R., Zalocusky, K.A., Zilberter, M., Yoon, S.Y., Hao, Y., Koutsodendris, N., Nelson, M., Rao, A., Taubes, A., Jones, E.A., Huang, Y. In vivo chimeric Alzheimer's disease modeling of apolipoprotein E4 toxicity in human neurons. Cell Rep., 32:107962, 2020; doi: 10.1016/j.celrep.2020.107962
Rao, A., Chen, N., Kim, M.J., Blumenfeld, J., Yip, O., Liang, Z., Shostak, D., Hao, Y., Nelson, M.R., Koutsodendris, N., Grone, B., Ding, L., Yoon, S.Y., Arriola, P., Zilberter, M., Huang, Y. Microglia depletion reduces human neuronal APOE4-related pathologies in a chimeric Alzheimer's disease model. Cell Stem Cell, 32:86-104.e7, 2025; doi: 10.1016/j.stem.2024.10.005
Raulin, A.C., Doss, S.V., Trottier, Z.A., Ikezu, T.C., Bu, G., Liu, C.C. ApoE in Alzheimer's disease: pathophysiology and therapeutic strategies. Mol. Neurodegener., 17:72, 2022; doi: 10.1186/s13024-022-00574-4
Rawat, V., Wang, S., Sima, J., Bar, R., Liraz, O., Gundimeda, U., Parekh, T., Chan, J., Johansson, J.O., Tang, C., Chui, H.C., Harrington, M.G., Michaelson, D.M., Yassine, H.N. ApoE4 alters ABCA1 membrane trafficking in astrocytes. J. Neurosci., 39:9611-9622, 2019; doi: 10.1523/JNEUROSCI.1400-19.2019
Sabir, M.S., Blauwendraat, C., Ahmed, S., Serrano, G.E., Beach, T.G., Perkins, M., Rice, A.C., Masliah, E., Morris, C.M., Pihlstrom, L., Pantelyat, A., Resnick, S.M., Cookson, M.R., Hernandez, D.G., Albert, M., Dawson, T.M., Rosenthal, L.S., Houlden, H., Pletnikova, O., Troncoso, J., Scholz, S.W. Assessment of APOE in atypical parkinsonism syndromes. Neurobiol. Dis., 127:142-146, 2019; doi: 10.1016/j.nbd.2019.02.016
Sepulveda, J., Kim, J.Y., Binder, J., Vicini, S., Rebeck, G.W. APOE4 genotype and aging impair injury-induced microglial behavior in brain slices, including toward Aβ, through P2RY12. Mol. Neurodegener., 19:24, 2024; doi: 10.1186/s13024-024-00714-y
Shi, Y., Yamada, K., Liddelow, S.A., Smith, S.T., Zhao, L., Luo, W., Tsai, R M., Spina, S., Grinberg, L.T., Rojas, J.C., Gallardo, G., Wang, K., Roh, J., Robinson, G., Finn, M.B., Jiang, H., Sullivan, P.M., Baufeld, C., Wood, M W., Sutphen, C., McCue, L., Xiong, C., Del-Aguila, J.L., Morris, J.C., Cruchaga, C.; Alzheimer’s Disease Neuroimaging Initiative; Fagan, A.M., Miller, B.L., Boxer, A.L., Seeley, W.W., Butovsky, O., Barres, B.A., Paul, S.M., Holtzman, D.M. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature, 549:523-527, 2017; doi: 10.1038/nature24016
Tsuang, D., Leverenz, J.B., Lopez, O.L., Hamilton, R.L., Bennett, D.A., Schneider, J.A., Buchman, A.S., Larson, E.B., Crane, P.K., Kaye, J.A., Kramer, P., Woltjer, R., Trojanowski, J.Q., Weintraub, D., Chen-Plotkin, A.S., Irwin, D.J., Rick, J., Schellenberg, G.D., Watson, G.S., Kukull, W., Nelson, P.T., Jicha, G.A., Neltner, J.H., Galasko, D., Masliah, E., Quinn, J.F., Chung, K.A., Yearout, D., Mata, I.F., Wan, J.Y., Edwards, K.L., Montine, T.J., Zabetian, C.P. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol., 70:223-8, 2013; doi: 10.1001/jamaneurol.2013.600
Wang, N., Wang, M., Jeevaratnam, S., Rosenberg, C., Ikezu, T.C., Shue, F., Doss, S.V., Alnobani, A., Martens, Y.A., Wren, M., Asmann, Y.W., Zhang, B., Bu, G., Liu, C.C. Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination. Mol. Neurodegener., 17:75, 2022; doi: 10.1186/s13024-022-00577-1
Yamazaki, Y., Painter, M.M., Bu, G., Kanekiyo, T. Apolipoprotein E as a therapeutic target in Alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs, 30:773-89, 2016; doi: 10.1007/s40263-016-0361-4
Zhao, N., Attrebi, O.N., Ren, Y., Qiao, W., Sonustun, B., Martens, Y.A., Meneses, A.D., Li, F., Shue, F., Zheng, J., Van Ingelgom, A.J., Davis, M.D., Kurti, A., Knight, J.A., Linares, C., Chen, Y., Delenclos, M., Liu, C.C., Fryer, J.D., Asmann, Y.W., McLean, P.J., Dickson, D.W., Ross, O.A., Bu, G. APOE4 exacerbates α-synuclein pathology and related toxicity independent of amyloid. Sci. Transl. Med., 12:eaay1809, 2020; doi: 10.1126/scitranslmed.aay1809