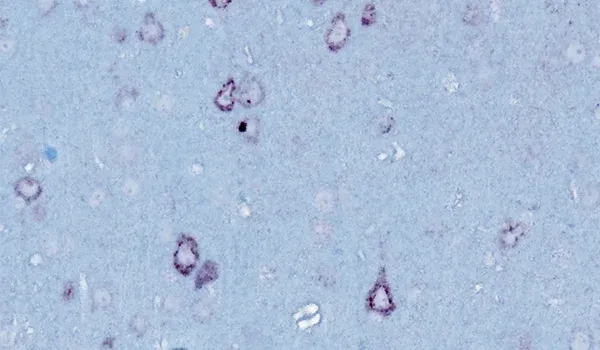
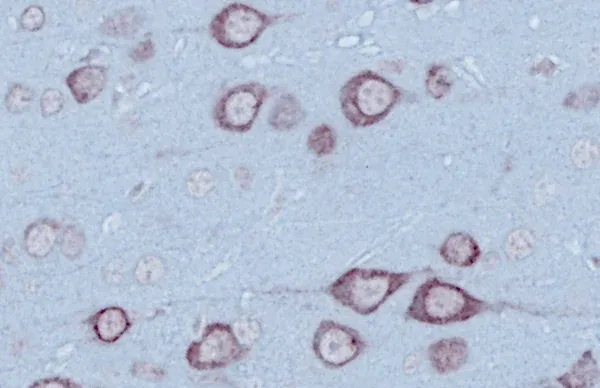
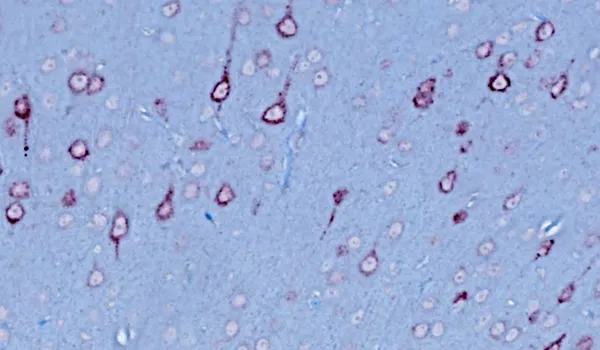
TDP-43转基因模型
细胞质TDP-43(TDP43;TARDBP)聚集是家族性和散发性肌萎缩侧索硬化症(ALS)的特征。我们使用TDP-43蛋白病的转基因rNLS8(或ΔNLS;delta NLS;dNLS)ALS模型(“TDP-43模型”)提供一系列服务。我们使用原始小鼠模型(“Off Dox”)或由Biospective开发的另一种进展较慢的版本(“Low Dox”)进行研究。前者可在数周内快速进展,后者进展较慢。
Off Dox和Low Dox模型均表现出TDP-43聚集物在细胞质中定位错误、运动功能逐渐丧失、运动神经元变性、大脑局部萎缩、神经炎症(小胶质细胞活化和反应性星形胶质细胞)、肌肉萎缩、CMAP改变以及大脑、脊髓和神经肌肉连接处病变。
我们的ALS模型可移植到人类疾病中
错误折叠的蛋白质聚集
TDP-43和SOD1等错误折叠的蛋白质聚集是散发性和家族性ALS的神经病理学特征。在>97%的ALS病例中,TDP-43聚集物被发现在大脑和脊髓神经元的细胞质中定位错误(Arnold,2023)。通常还会观察到磷酸化聚集。这些特征在我们的TDP-43ΔNLS小鼠模型中很容易观察到。
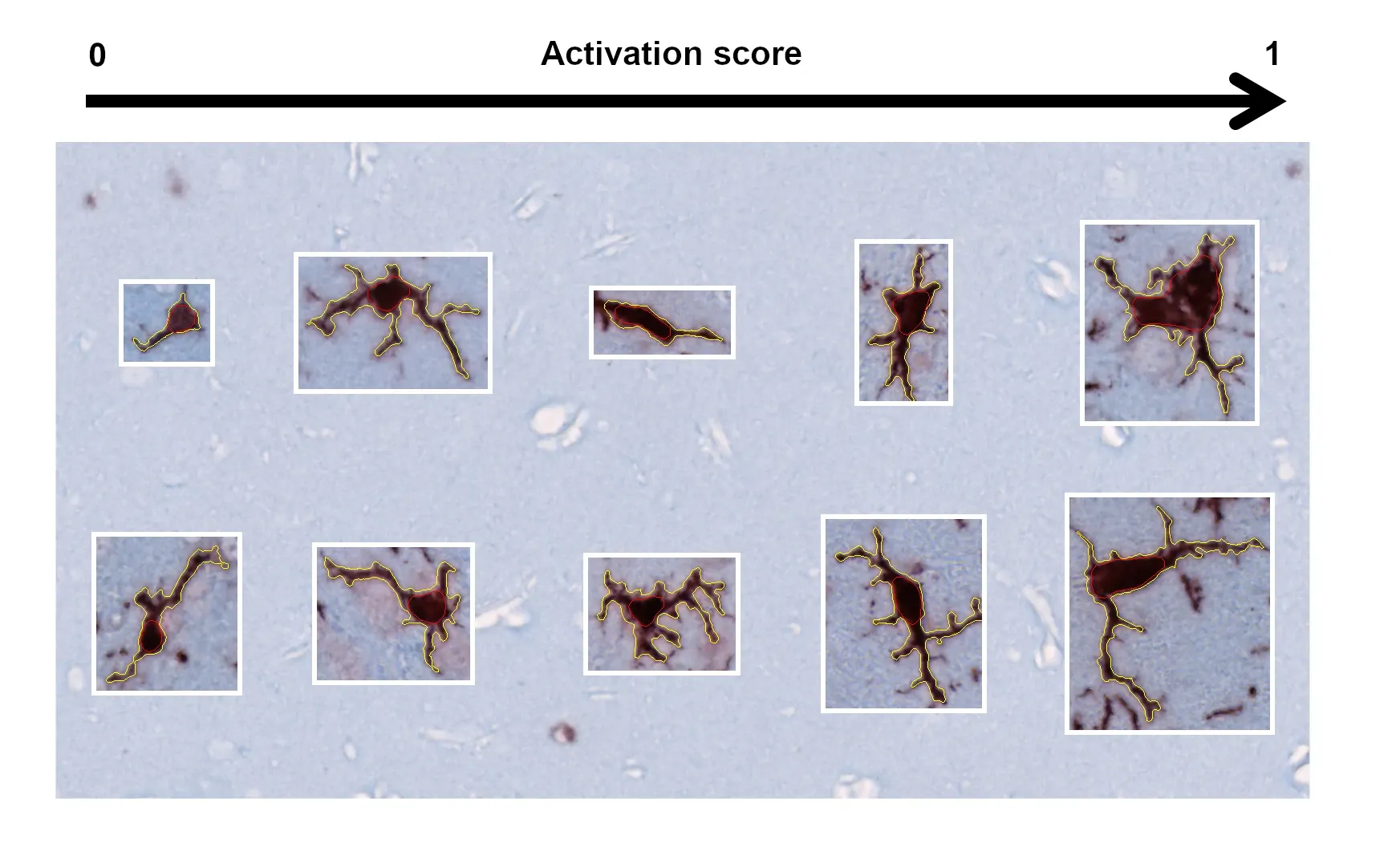
活化的微胶质细胞和反应性星形胶质细胞
活化的微胶质细胞和反应性星形胶质细胞是ALS患者大脑和脊髓中突出的神经炎症特征,被认为在疾病发病机理中起着关键作用(Clarke和Patani,2020 ;Yang,2024)。我们观察到TDP-43小鼠模型中神经炎症随时间推移而增加。除了Iba-1和GFAP免疫反应性增强外,我们还发现,使用我们开发的算法测量小胶质细胞和星形胶质细胞的形态,激活与运动表型之间存在密切关系。
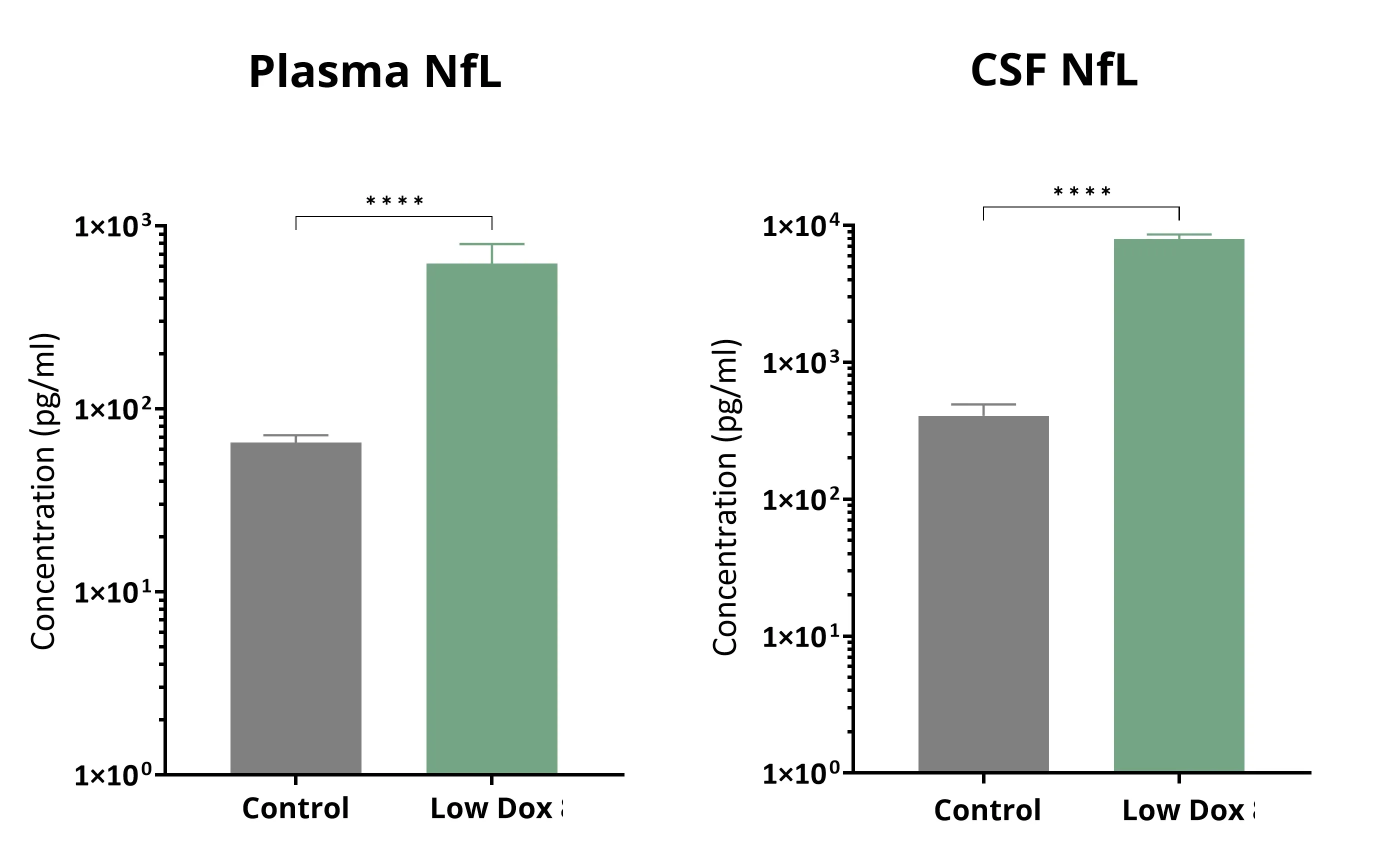
血浆和脑脊液中神经丝轻链升高
ALS患者血浆和脑脊液中的神经丝轻链含量增加(Benatar,2023)。神经丝轻链测量在ALS临床试验中经常使用。托法森(Qalsody)加速获批,同时神经丝轻链水平降低,这表明FDA认可该测量作为疾病生物标志物。我们观察到TDP-43小鼠模型中血浆和脑脊液中的神经丝轻链水平显著增加。 Young等人已经证实 ,一种名为AIT-101(INN:阿匹莫德,又名LAM-002A)的小分子PIKfyve抑制剂能够降低我们的“低剂量多柔比星”TDP-43ΔNLS模型中的神经丝轻链水平。
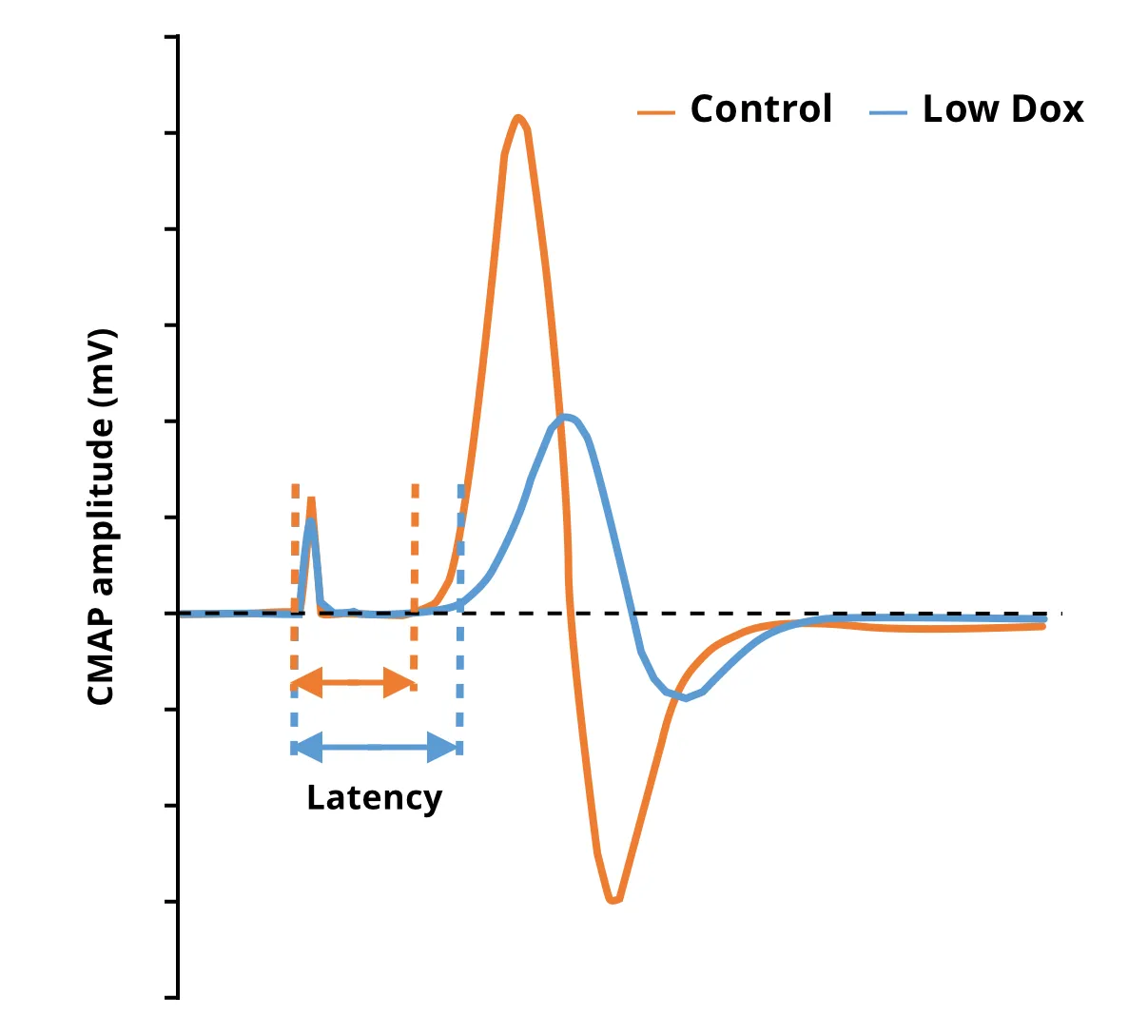
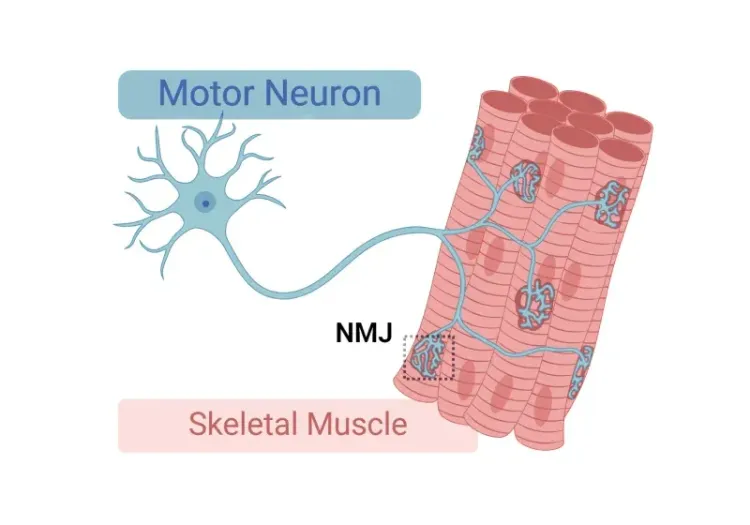
NMJ 神经去神经化和形态改变
电生理学(例如肌电图[EMG])是ALS患者诊断和监测疾病的标准测试。复合肌肉动作电位(CMAP)等肌电图测量值降低反映了由于脊髓运动神经元丢失导致的神经肌肉神经支配功能丧失(Sleutjes,2021)。在ALS患者的肌肉中也发现了神经肌肉接头(NMJ)的神经支配和形态变化(Bruneteau,2015)。在我们的“低剂量多柔比星”TDP-43ΔNLS模型中,我们发现CMAP振幅明显降低,潜伏期延长。使用多重免疫荧光,我们还发现了与神经支配一致的NMJ改变。
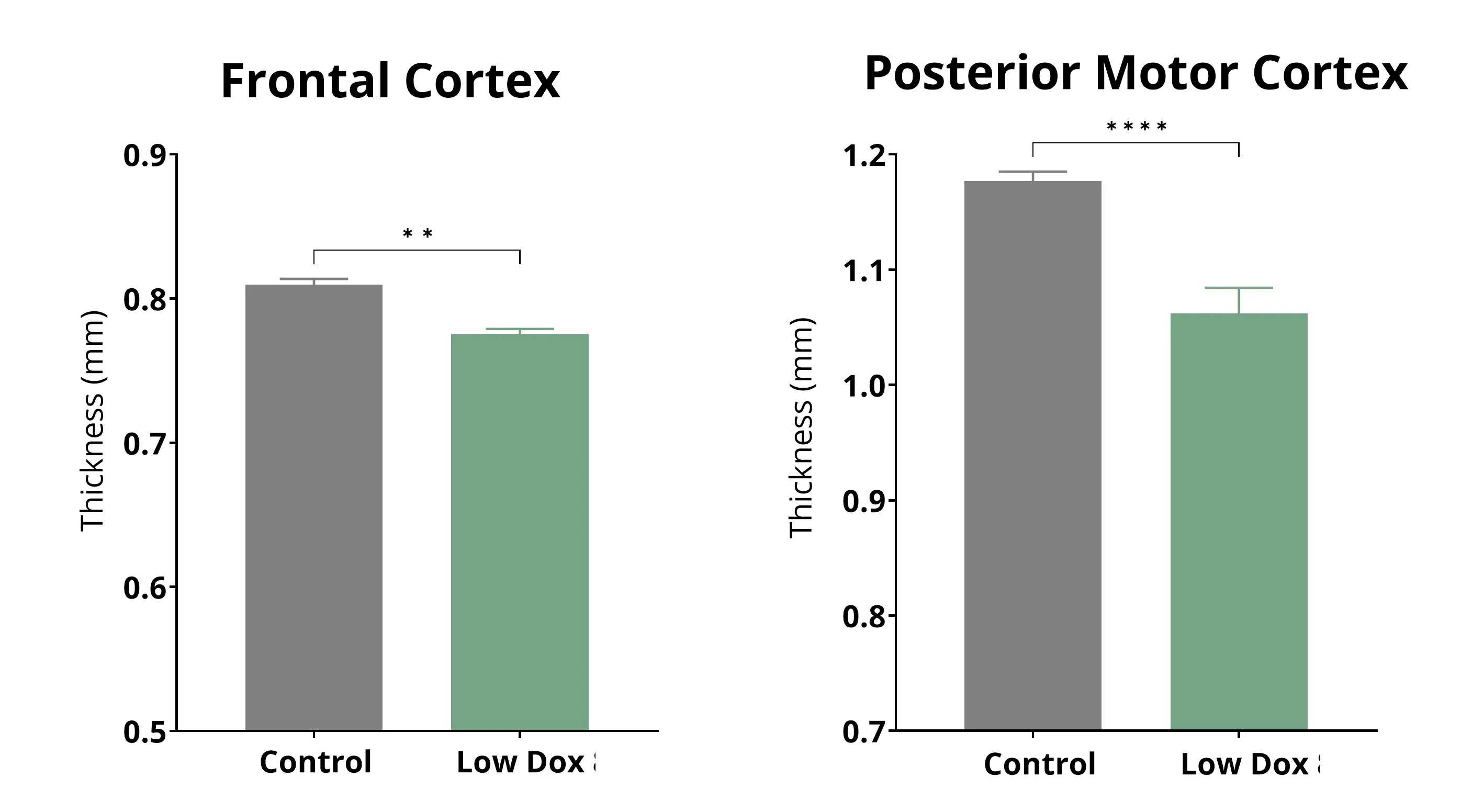
区域性脑萎缩
神经影像生物标记广泛用于包括ALS在内的神经退行性疾病的临床试验。MRI衍生的区域体积和皮质厚度测量对脑萎缩高度敏感,可用于监测疾病随时间推移的进展。ALS的运动和非运动脑区均出现皮质变薄(Yang,2025)。通过高分辨率全脑MRI采集和全自动图像处理与分析,我们证明了低剂量多柔比星TDP-43ΔNLS小鼠模型中可重复的大脑萎缩(尤其是运动和额叶皮层),从而成为神经变性的一种可靠的活体测量方法,可作为液体神经丝轻链测量等其他方法的补充。
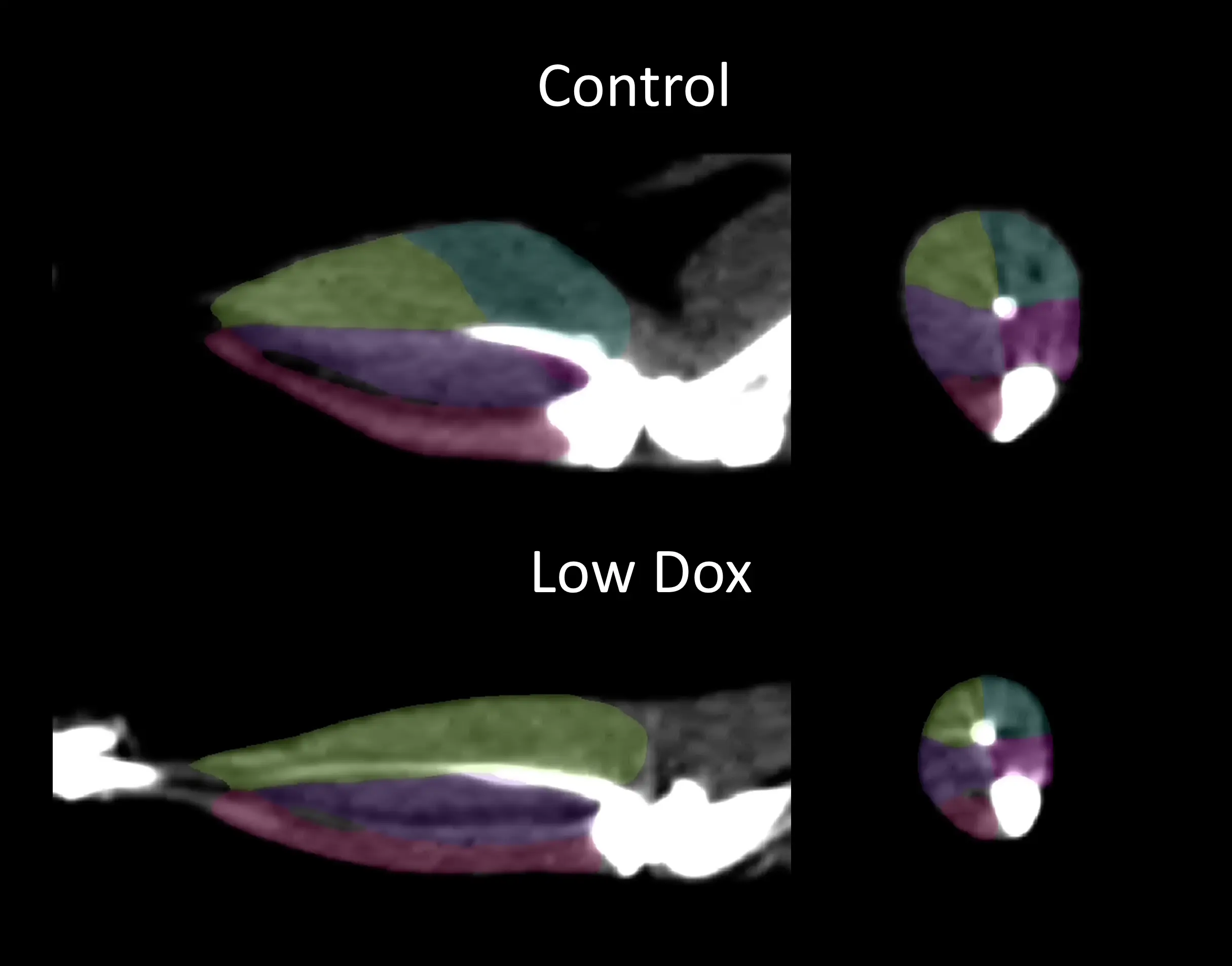
肌肉萎缩和无力
骨骼肌萎缩和无力是ALS的主要临床特征(Shefner,2023)。非侵入性成像技术已被用于量化ALS患者的肌肉萎缩(Jenkins,2013;Jenkins,2018 ;Wilcox,2021;Klickovic,2024)。我们使用微CT对低剂量多柔比星TDP-43ΔNLS小鼠模型的后肢肌肉萎缩进行了纵向测量 ,发现与对照组小鼠相比差异非常显著。这种肌肉萎缩伴随着握力计测量的肌肉力量下降。
了解更多关于我们对这些ALS小鼠模型的表征、我们经过验证的测量方法以及我们的临床前神经科学合同研究组织服务。
相关内容
ALS的最新信息以及ALS动物模型治疗剂评估的最佳实践。
神经肌肉接头(NMJ)形态学与ALS模型
对神经肌肉接头(NMJ)及其在肌萎缩侧索硬化症(ALS)中作用的深入理解,以及用于研究NMJ形态学变化的工具和方法。
ALS 小鼠模型和脊髓运动神经元
脊髓运动神经元在小鼠肌萎缩侧索硬化症(ALS)模型中疾病进展的概述。
ALS小鼠模型用于药物研发
指导如何最有效地使用肌萎缩侧索硬化症(ALS)的实验动物模型(小鼠和大鼠模型)进行临床前治疗测试。
TDP-43 ΔNLS (rNLS8) 小鼠用于ALS药物研发
该资源提供了有关使用ΔNLS(deltaNLS、hTDP-43ΔNLS、hTDP-43DeltaNLS、dNLS、TDP43 NLS、rNLS8)TDP-43 ALS转基因小鼠模型进行临床前治疗研究的信息。
ALS、阿尔茨海默氏症和帕金森氏症中的微胶质形态
概述微胶质形态学分析及其在神经退行性疾病研究和药物研发中的应用。