淀粉样蛋白β转基因模型
人类阿尔茨海默病的典型病理——β-淀粉样蛋白,可通过转基因小鼠中人类淀粉样蛋白前体蛋白(APP)和早老性痴呆症1(PS1;PSEN1)的突变体过量表达来模拟。与人类疾病类似,病理的演变会随着年龄的增长而加剧。
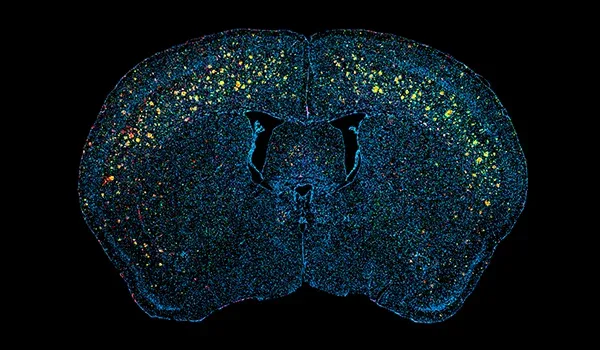
我们用于临床前评估实验性、疾病改良治疗剂疗效的APP/PS1模型具有高度可重复性,并复制了人类AD的多个关键特征。这些小鼠表现出淀粉样蛋白(Aβ)斑块、脑血管病变和神经炎症的渐进性发展。治疗干预的反应可以通过多种定量读数进行评估,包括对数字化脑组织切片的多重免疫荧光染色进行高级图像分析。
淀粉样β与tau蛋白共病理模型
我们的淀粉样β(Aβ)和tau共病理小鼠模型通过结合转基因和病毒载体技术,成功再現了阿尔茨海默病(AD)的两大核心特征——Aβ斑块沉积和tau相关神经退行性变。该模型为研究疾病机制及评估阿尔茨海默病疾病修饰疗法的有效性提供了强大且具有临床转化潜力的研究平台 。
淀粉样β病理通过APP/PS1转基因小鼠建立,该模型表现出年龄依赖性的Aβ斑块沉积。为诱导tau病理,通过立体定向注射将携带野生型人类tau(2N4R)基因的腺相关病毒(AAV)载体递送至与疾病相关的脑区。这种靶向表达导致磷酸化tau聚集物在神经元胞体及突触中积累。该共病理模型显示出显著的神经炎症、神经退行性变及相关功能障碍,反映了与阿尔茨海默病相关的复杂病理相互作用。
阿尔茨海默氏症和Tau蛋白病模型对人类疾病的可转化性
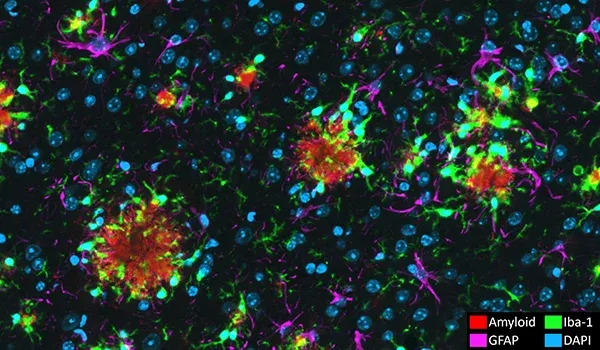
淀粉样蛋白斑块和脑血管病变
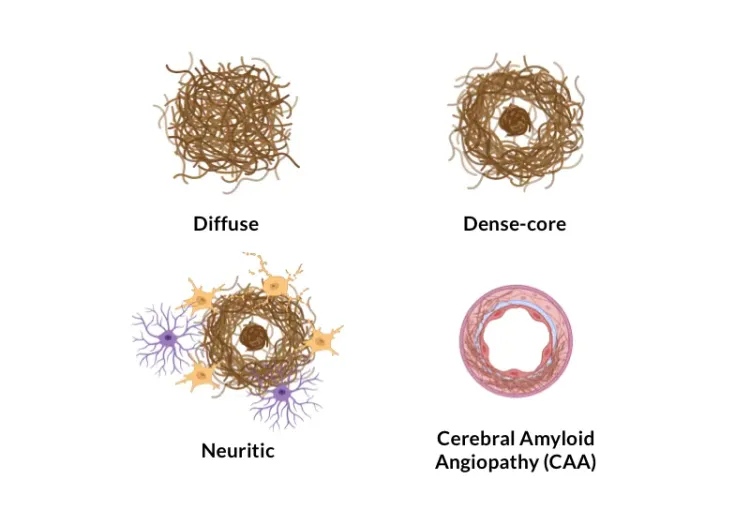
淀粉样蛋白β聚集形成的细胞外斑块和脑血管沉积是阿尔茨海默病的神经病理学特征(Serrano-Pozo,2011)。我们的APP/PS1小鼠模型显示淀粉样蛋白病理(包括弥漫性、致密核和神经斑块、细胞内淀粉样蛋白和脑血管病理)随时间推移而增加。淀粉样蛋白病理以一种明确的时空模式发展,并可通过我们团队开发的复杂算法进行量化。
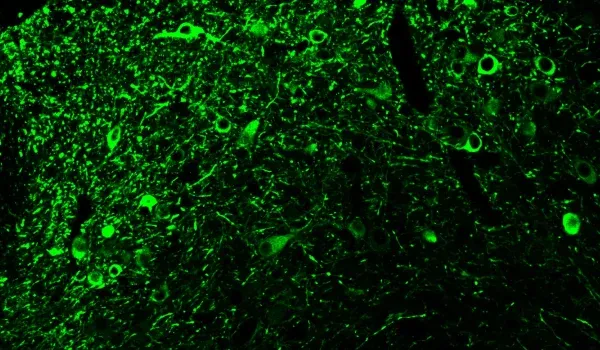
Tau 病理学
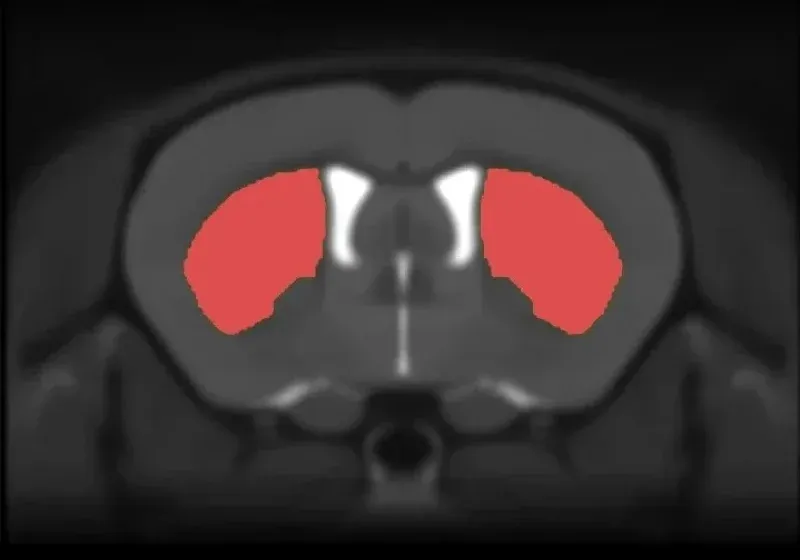
此外,淀粉样蛋白和β蛋白、Tau蛋白是阿尔茨海默病中的一种关键错误折叠蛋白。Tau蛋白被认为是AD(Lew,2021;Carbonell,2025)一些临床和神经影像学特征的主要驱动因素。我们的APP/PS1/人类Tau模型同时展示了淀粉样蛋白和β蛋白以及Tau蛋白的病理学。在细胞体和突起中观察到磷酸化tau染色。 进行性核上性麻痹、皮质基底变性、额颞痴呆等tau病在特定脑区表现出纯tau病理。 在我们的AAV-hTau模型中,我们能够将tau表达定位于黑质和中脑区域,从而有效地模拟具有帕金森特征的tau病。
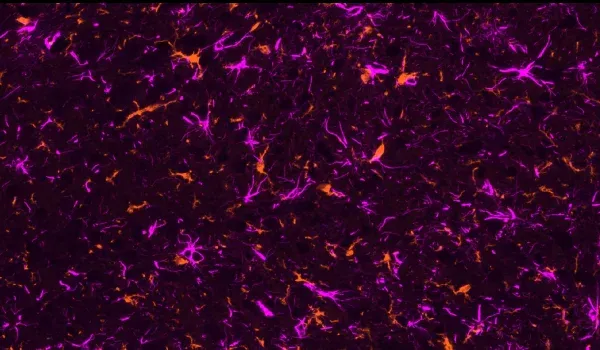
活化的微胶质细胞和反应性星形胶质细胞
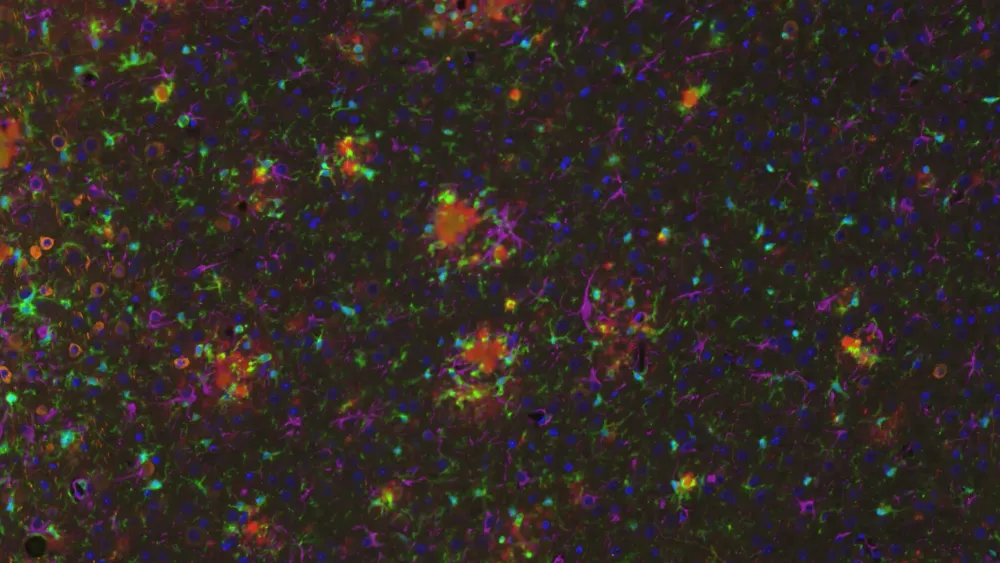
神经炎症细胞,包括活化的微胶质细胞和反应性星形胶质细胞,与错误折叠的淀粉样蛋白和tau蛋白紧密相邻(Minter,2015 ;Chen和Yu,2023)。在我们的APP/PS1小鼠模型中,我们证明了Aβ斑块、活化小胶质细胞和非活化小胶质细胞之间以及 Aβ斑块和肥大&非肥大星形胶质细胞之间存在空间和时间关系。在我们的APP/PS1/hTau共病模型和AAV诱导的tau病模型中,我们还观察到与磷酸化tau相关的强烈小胶质细胞和星形胶质细胞病变。
Tau病 帕金森运动特征
进行性核上性麻痹和皮质基底变性是纯粹的tau病,除其他临床表现外,其特点为帕金森氏症特征和黑质神经元严重丢失(Oyangi,2001)。在我们的AAV-hTau模型中,我们发现由于黑质多巴胺能神经元退化和相应的纹状体神经元缺失,出现了明显的运动障碍(基于圆柱测试、悬尾摆动测试、旋转棒测试和后肢夹紧测试)。
阿尔茨海默病小鼠模型特点
通过下面的互动演示,您可以探索人类 AD 和阿尔茨海默病小鼠模型中淀粉样蛋白-β、tau 和脑萎缩之间的关系,包括整个多重免疫荧光组织切片的活体数据和高分辨率图像。
您只需使用左侧面板浏览“图像故事”即可。
您可以使用鼠标左键在高清显微镜图像中平移。您可以使用 鼠标/触控板(上/下)或左上角的+和-按钮放大和缩小 。您可以在右上角的控制面板中 切换(开/关)、更改颜色以及调整通道和分割的图像设置。
我们建议使用 全屏模式,以获得 最佳交互体验。
了解更多关于我们阿尔茨海默氏病和Tau蛋白病小鼠模型的特征、我们经过验证的测量方法以及我们的临床前神经科学合同研究组织(CRO)服务。
相关内容
关于阿尔茨海默氏病和阿尔茨海默氏病动物模型治疗剂评估最佳实践的最新信息。
ALS、阿尔茨海默氏症和帕金森氏症中的微胶质形态
概述微胶质形态学分析及其在神经退行性疾病研究和药物研发中的应用。
阿尔茨海默氏症中的淀粉样蛋白斑块分析
人类和阿尔茨海默病动物模型(转基因小鼠和大鼠)脑组织切片中的Aβ斑块分类和量化方法概述。