Characterization of a New Amyloid-β & Tau Co-Pathology Mouse Model of Alzheimer's Disease
淀粉样β与tau蛋白共病理小鼠模型(APP/PS1/hTau) 概述
对于该阿尔茨海默病模型,我们 对双转基因APP/PS1小鼠的多个脑区进行多次双侧立体定向注射,表达 过量AAV-hTau(野生型2N4R人tau)的腺相关病毒(AAV) 。
该小鼠模型再現了人类阿尔茨海默病的多项关键特征,包括:
- β-淀粉样斑块
- 细胞体和神经突中磷酸化tau蛋白的聚集
- 活化的微胶质细胞
- 反应性星形胶质细胞
- 睡眠障碍
- 区域性脑萎缩(体积和皮层厚度)通过活体MRI扫描测量
淀粉样蛋白 -β与tau蛋白共病理小鼠模型(APP/PS1/hTau)的建立
该模型建立的一般流程图如下:
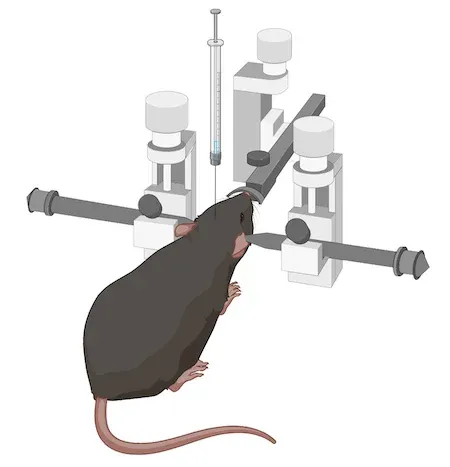
对于该特定模型,我们通常使用约6个月大的APP/PS1小鼠(尽管也可使用更年轻或更年老的小鼠)。随后,我们通过立体定向注射将AAV载体注入多个与疾病相关的脑区。我们采用配备自动微注射器的数字立体定向设备,以确保高精度与高准确性。
研究的体内阶段 通常持续4-12周。在体内的读出指标,如MRI脑萎缩,最早可在4周时显示显著效果。因此,读出指标的生成可在相对较短的时间内完成,尤其与传统阿尔茨海默病及tau病变的tau转基因模型相比。
模型特征分析
下方的互动演示文稿使您能够探索我们对淀粉样β蛋白(β-淀粉样蛋白)与人类tau蛋白共病理(APP/PS1/hTau)小鼠模型的特征化研究,包括体内实验数据及高分辨率的多重免疫荧光组织切片图像。
您可通过左侧面板在该“图像故事”中自由浏览。
您可以使用鼠标左键在高分辨率显微镜图像中自由移动。您可以通过 鼠标/触控板(上下滚动)或左上角的“+”和“-”按钮进行缩放 。您可以在右上角的控制面板中 切换(开启/关闭)、更改颜色以及调整通道和分割的图像设置。
建议使用 全屏模式以获得 最佳互动体验。
1/12
Alzheimer’s disease (AD) is pathologically defined by the presence of amyloid-β plaques and tau neurofibrillary tangles. While a broad range of animal models of AD exist, these models typically demonstrate amyloid-β or tau pathology, but not both. As such, there is a need for a “co-pathology” model which better recapitulates human disease and demonstrates features that can be measured using “translational biomarkers”.
Our group has developed an adeno-associated virus (AAV) vector-induced mouse model of tauopathies with Parkinsonian features (e.g. Progressive Supranuclear Palsy, Corticobasal Degeneration). We have adapted this modeling strategy by injecting AAV-hTau into a transgenic APP/PS1 mouse model to generate a co-pathology model of AD.
This Interactive Presentation illustrates some of the interesting behavioral, neuroimaging, and pathologic features of Biospective's amyloid-β/hTau co-pathology mouse model.
This model was generated by injecting 6 month-old transgenic APP/PS1 (ARTE10) mice with AAV-hTau (wild-type 2N4R human tau) or AAV-null (control) vectors bilaterally into the anterior insula and the lateral entorhinal cortex using a digital stereotaxic device with an automated microinjector.

Atlas Views of Cortical Injection Sites of AAV-Tau vectors
Multiplex immunofluorescence (mIF) images were generated by immunostaining for amyloid-β (fibrillar), phospho-tau (AT8), conformationally altered tau (MC1), oligomeric tau (T22), GFAP, Iba-1, and counterstained with the DAPI nuclear stain. Tissue sections were digitized using a high-throughput slide scanner and were processed using Biospective's PERMITSTM software platform.
To navigate though this Image Story, you can use the arrows and/or the Table of Contents icon in the upper right corner of this panel.

You can also interact with the microscopy image in the viewer on the right at any time to further explore this high-resolution data.
Overview of the APP/PS1 (ARTE10) Transgenic Mouse Model
ARTE10 [C57BL/6NTac.CBA-Tg(Thy1-PSEN1*M146V,-APP*Swe)10Arte] (APP/PS1) homozygous mice (Willuweit, 2009), generated on a C57BL/6NTac background, are a transgenic line incorporating the Swedish mutation of human amyloid precursor protein (APPsw) and the M146V mutation in human Presenilin 1 (PS1M146V). These mice express high levels of human amyloid-beta (Aβ) peptides via amyloidogenic processing of APP, and develop Alzheimer's disease-like amyloid pathology. This transgenic mouse model has been used for non-invasive imaging of amyloid-β plaques with Amyloid PET imaging tracers (Willuweit, 2021).

Representative coronal brain tissue sections showing the spatiotemporal progression of amyloid-β pathology in APP/PS1 (ARTE10) mice.

Quantitative analysis of the age-dependent increase in the density of amyloid-β plaques in the cerebral cortex of APP/PS1 (ARTE10) mice. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Our team at Biospective has also characterized the neuroinflammatory microenvironment around plaques in this model, as well as examined both microglia morphology and astrocyte morphology.

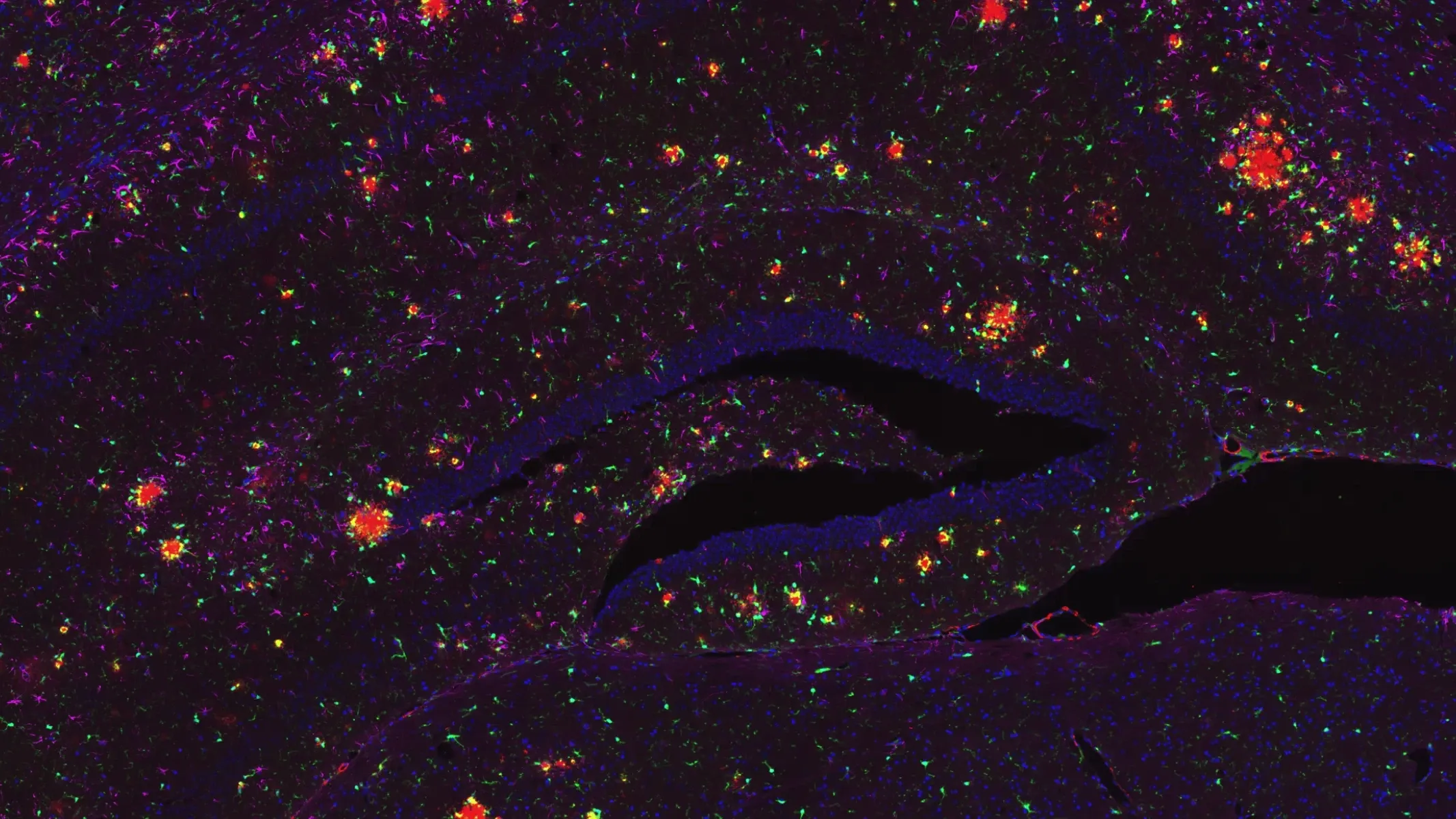
Examples of “neighborhoods” of amyloid-β plaques to allow for microenvironment analysis.
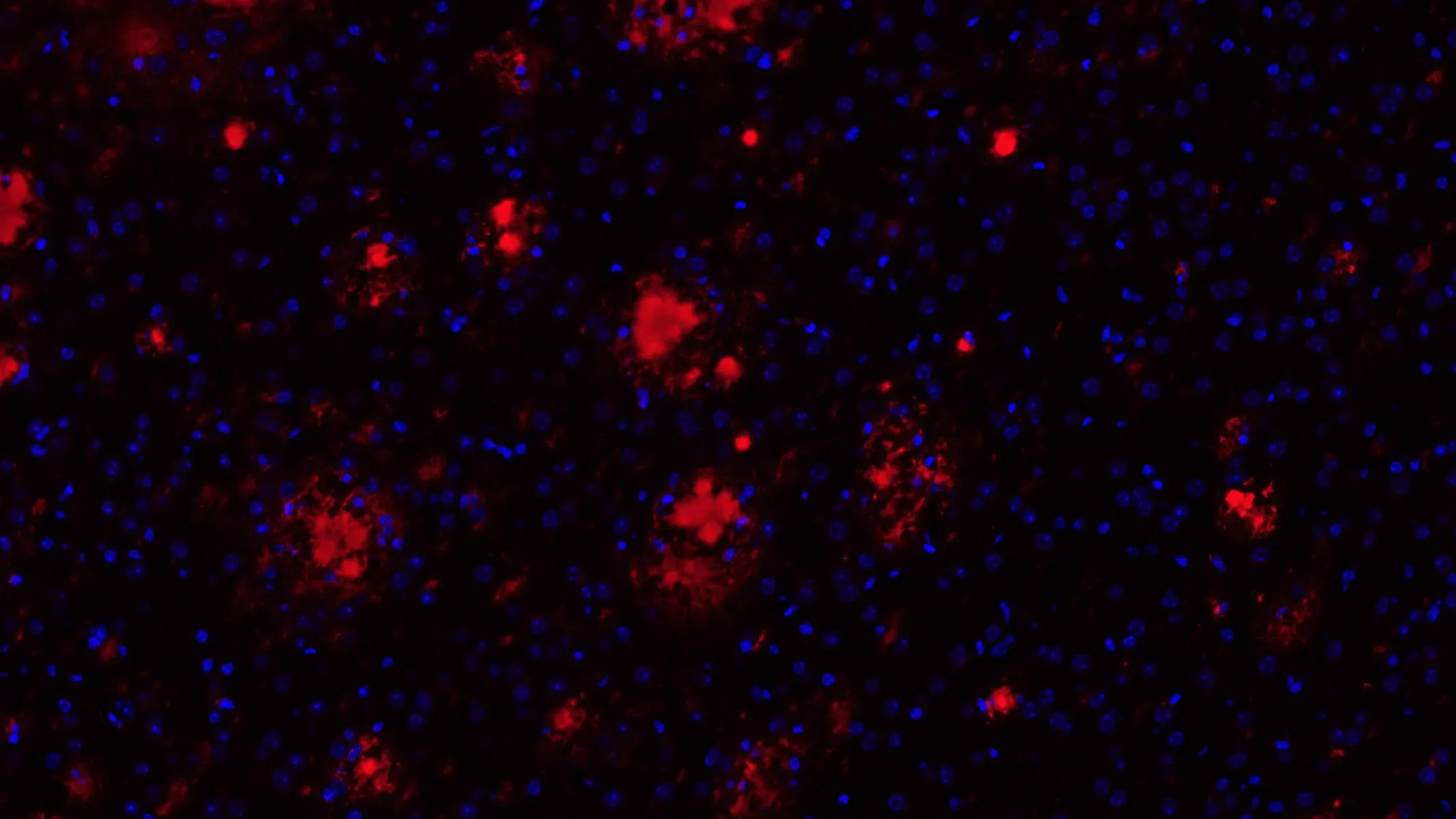
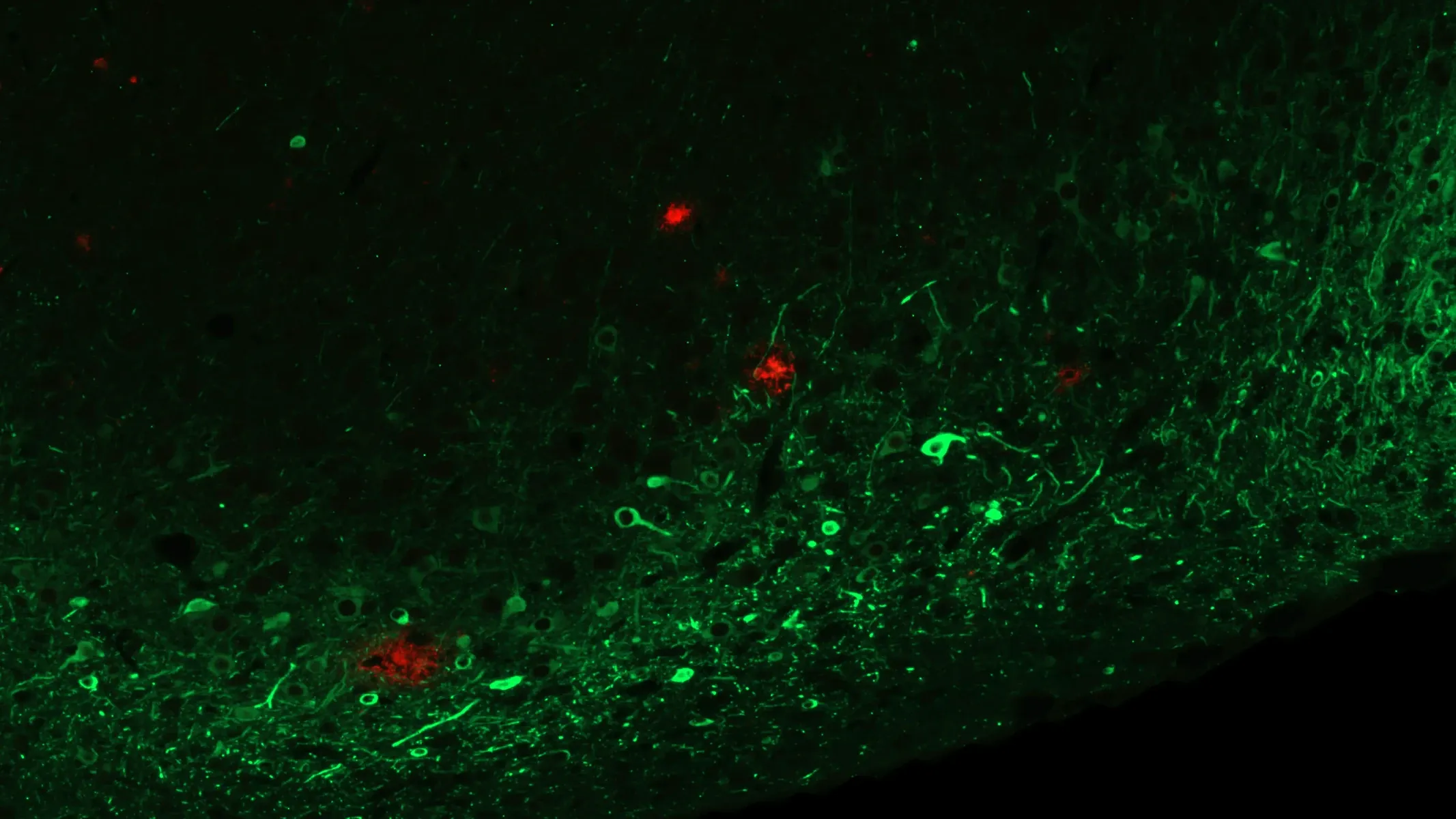
Amyloid-β and Phosphorylated Tau in APP/PS1/hTau Mice (Middle Brain; Low Magnification)
Low magnification image showing phosphorylated tau (in neuronal soma and processes) and fibrillar amyloid-β (plaques and vascular pathology). Note the extensive phosphorylated tau in the piriform cortex. For reference, an illustration with atlas labels for this approximate brain level is provided below.

Coronal Mouse Brain Section (Bregma -1.0) with Neuroanatomy Labels
Amyloid-β and Phosphorylated Tau in APP/PS1/hTau Mice (Middle Brain; High Magnification)
High magnification image showing phosphorylated tau (in neuronal soma and processes) and fibrillar amyloid-β (plaques and vascular pathology). Note the extensive level of phosphorylated tau in the piriform cortex. Similar pathology is also observed in the anterior cortex (not shown).
Pathological Tau Accumulation in APP/PS1/hTau Mice (Middle Brain; Low Magnification)
Low magnification image showing MC1 and T22 immunostaining reveals the distribution of tau pathology across distinct brain regions. Accumulation of conformationally altered tau (MC1) and oligomeric tau (T22) was observed in the cell soma and neurites in various brain regions of AAV-Tau-injected mice, both at the injection site and in connected distal areas.
MC1-Positive Misfolded Tau in Piriform Cortex of APP/PS1/hTau Mice (Middle Brain; High Magnification)
High magnification image of the piriform cortex stained with MC1 antibody. Prominent accumulation of misfolded tau indicates early pathological changes. Similar staining was observed at the level of the anterior insula and the lateral entorhinal cortex.
Tau Oligomer Accumulation in Piriform Cortex (T22) of APP/PS1/hTau Mice (Middle Brain; High Magnification)
High magnification image of T22 staining in the piriform cortex reveals accumulation of tau oligomers. Similar staining was observed at the level of the anterior insula and the lateral entorhinal cortex.
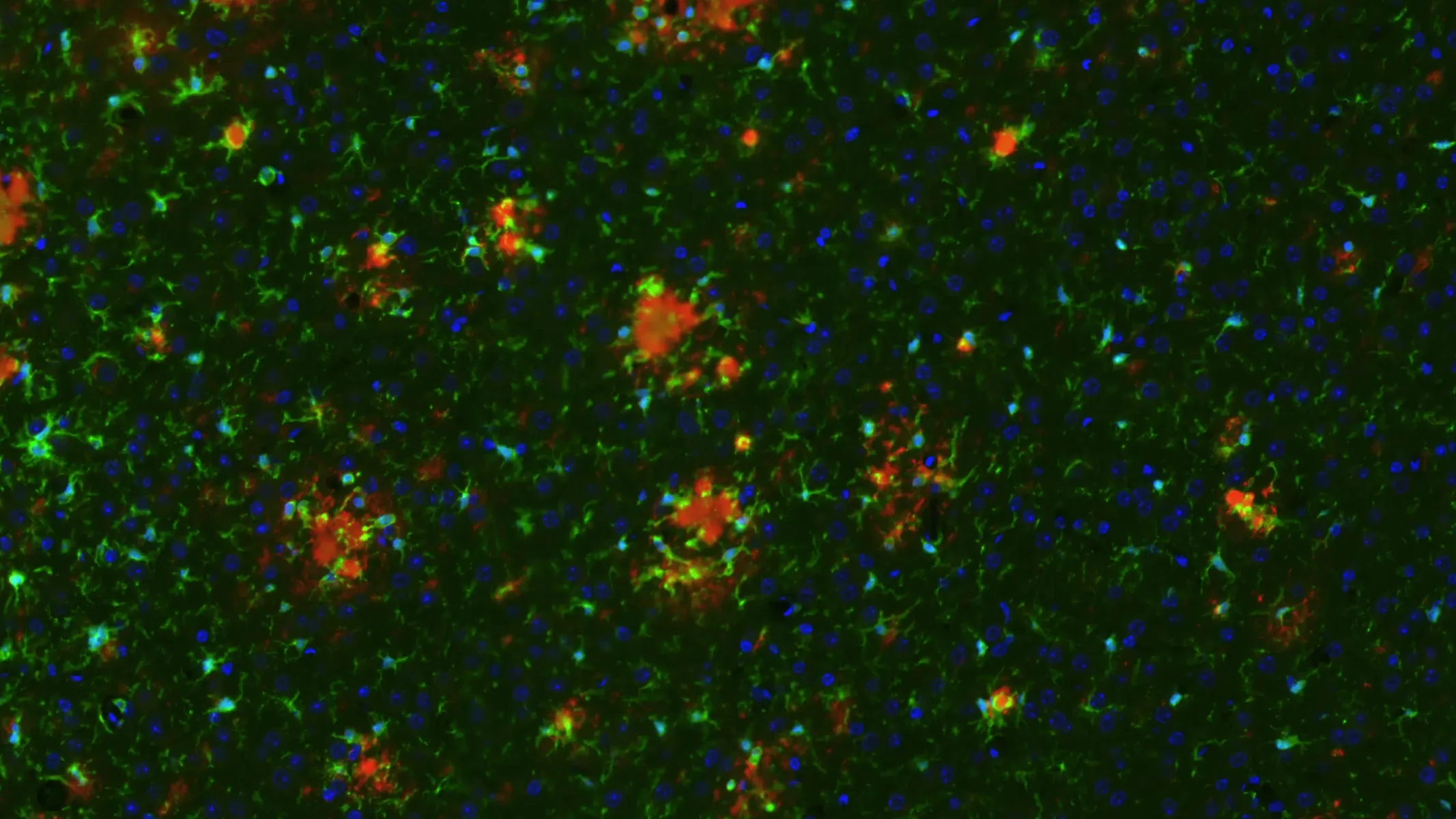
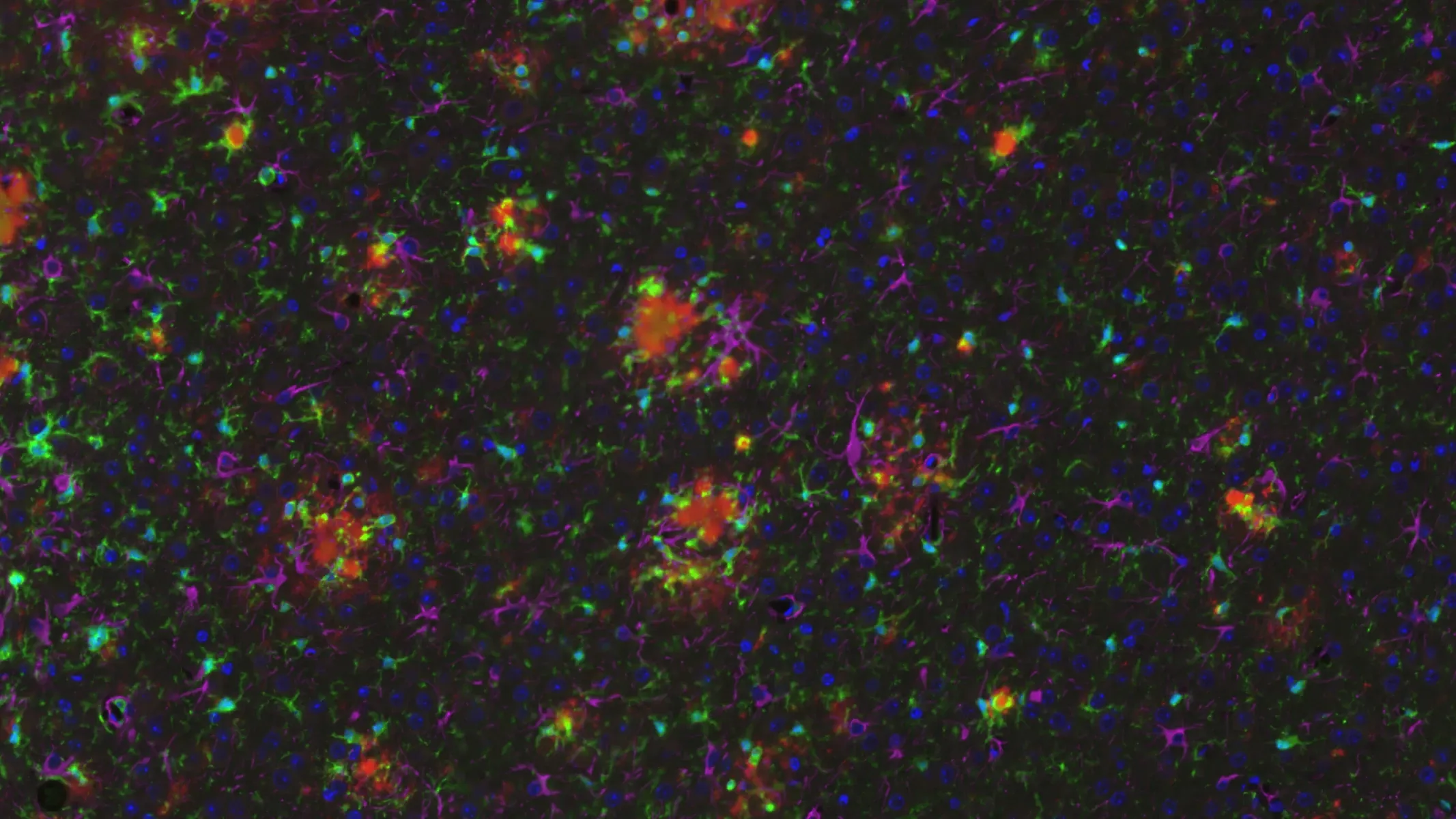
pTau, Microgliosis, and Astrogliosis in APP/PS1/hTau Mice (Middle Brain; High Magnification)
High magnification image showing phosphorylated tau (in neuronal soma and processes), microglia, and astrocytes. Note the extensive level of neuroinflammation in the piriform cortex.
The plots below show the quantitative analysis of Iba-1 and GFAP stain density in brain regions with amyloid-β and tau pathology.

Iba-1 stain density for APP/PS1/hTau compared to APP/PS1 (control) mice in Anterior, Piriform, and Entorhinal Cortex regions; mean ± SEM, t-test, *** p<0.001

GFAP stain density for APP/PS1/hTau compared to APP/PS1 (control) mice in Anterior, Piriform, and Entorhinal Cortex regions; mean ± SEM, t-test, *** p<0.001, ****p<0.0001
Sleep Alterations in APP/PS1/hTau Mice
Sleep is altered in Alzheimer’s disease and has been associated with tau-driven neuropathology. Increased daytime sleep has been observed in later stages of the disease.
We have performed an assessment of sleep-wake cycles in the APP/PS1/hTau model using the non-invasive PiezoSleep system. The plot below shows the increased level of sleep in the dark phase in APP/PS1/hTau mice compared to APP/PS1 mice (corresponding to daytime sleep in humans).

Percentage of sleep in the light and dark phases measured by the PiezoSleep system.
Brain Atrophy in the APP/PS1/hTau Model
We have acquired in vivo anatomical MRI data from wild-type (WT), WT/hTau, APP/PS1, and APP/PS1/hTau mice at 4 weeks following injection of AAV-hTau or AAV-null (control) vectors. We generated regional volumes and cortical thickness measures using our fully-automated NIGHTWINGTM image processing platform. The figures below show MRI atlases and quantitative measures in several brain regions.

Anatomical MRI with segmented regions, and plots of regional volumes assessed in wild-type (hashed), and APP/PS1 (solid), AAV-null and hTau mice. **p<0.01,***p<0.001, ****p<0.0001

Mouse brain surface rendering with segmented entorhinal cortex, as well as a plot of the regional thickness assessed in wild-type (hashed), and APP/PS1 (solid), AAV-null and hTau mice. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Note that APP/PS1 mice do not show any brain atrophy compared to WT mice. The injection of AAV-hTau induced highly significant reductions of regional volumes and cortical thickness. Interestingly, the APP/PS1/hTau mice appear to have greater brain atrophy compared the the WT/hTau mice, suggesting a potential modulatory role of amyloid-β.
Translation of Mouse MRI Brain Atrophy Data to Human Alzheimer's Disease
Our team at Biospective has performed a rigorous analysis of the relationship between amyloid-β, tau, and cortical thickness in human Alzheimer’s disease. This analysis was performed using Amyloid PET, Tau PET, and 3D Anatomical MRI data from the ADNI study. We have found that tau, rather than amyloid-β, is primarily responsible for cortical thinning, as well as regional cerebral glucose metabolism, which can be appreciated in the figure below.

t-Statistic maps (thresholded for statistical significance) demonstrating the effect of tau and amyloid-β on both cortical thickness and cerebral glucose metabolism.
We have further demonstrated that the correlation between tau and cortical thickness is increased as the amyloid-β burden increases, which is apparent in the video below.
Statistical maps showing increased regional correlation between tau and cortical thickness as a function of amyloid-β load.
This human neuroimaging data corresponds well with our mouse MRI data showing that tau is the primary driver of brain atrophy with an apparent increase in the presence of amyloid-β.
Summary
This novel amyloid-β/tau co-pathology mouse model recapitulates several features of Alzheimer’s disease. In terms of the neuropathology, we have observed parenchymal (including diffuse, dense-core, and neuritic plaques) and vascular Aβ aggregates, phosphorylated tau in cell bodies and processes (including dystrophic neurites), microgliosis, and astrogliosis. We plan to further explore the relationships between the misfolded proteins and neuroinflammation in this model.
One of the most interesting observations is the neurodegenerative phenotype in the APP/PS1/hTau mice. The regional brain atrophy observed via structural analysis of the anatomical MRI scans can provide a robust way to evaluate the effects of potential interventions and serve as a translational biomarker given the widespread use of neuroimaging in AD clinical trials.
Based on the quantifiable in-life and post-mortem measures that we have reported, APP/PS1/hTau mice can serve as a useful model for preclinical evaluation of novel disease-modifying therapeutics for Alzheimer’s disease.
Please feel free to further explore the microscopy image in the viewer.
We would be happy to discuss this model and our characterization if you would like to Contact Us.
了解更多关于我们对APP/PS1/hTau模型的特征分析、经过验证的测量方法以及我们的临床前神经科学合同研究组织(CRO)服务。
探索更多关于阿尔茨海默病与tau病变的模型
在这种β-淀粉样蛋白与tau蛋白共病理模型中是否观察到脑萎缩?
是的。 最有趣的观察结果之一是APP/PS1/hTau小鼠的神经退行性表型。通过 对 解剖学MRI扫描的结构分析所观察到的区域性脑萎缩(体积和皮层厚度)可为评估潜在干预措施的效果提供一种可靠的方法,并可作为转化生物标志物,因为神经影像学在阿尔茨海默病临床试验中广泛应用。
在 这种β-淀粉样蛋白与tau蛋白共病理模型中,随着 病理的进展 ,是否 观察到 生理变化 ?
是的。 使用非侵入 性 PiezoSleep系统 对APP/PS1/hTau模型进行 睡眠结构分析显示,与APP/PS1小鼠相比, APP/PS1/hTau小鼠 在黑暗阶段的睡眠时间显著增加,这 与 人类 的白天 睡眠相一致 。值得注意的是,睡眠障碍与阿尔茨海默病的发展相关, 并已被证实与tau蛋白驱动的神经病理学改变有关。白天睡眠时间的增加 在疾病晚期尤为 明显 。
该模型 是否可应用于 人类阿尔茨海默病?
是的。 基于MRI的脑萎缩定量分析和PET葡萄糖代谢评估提供了有价值的转化生物标志物,将动物研究与人类临床试验相连接。Biospective已开发出用于评估阿尔茨海默病及其他神经退行性疾病(如进行性核上性麻痹、皮层基底节变性及额颞叶痴呆 ) 中MRI和PET的分析流程 。
我们已发表多篇资源和创新报告,详细描述了MRI和PET生物标志物的应用,包括 :
- tau相关萎缩与β-淀粉样蛋白及APOE ε4无关(创新报告 )
- 轻度认知障碍中脑葡萄糖代谢降低由tau驱动(创新报告 )
- 轻 度 认知障碍(MCI)与阿尔茨海默病中tau PET的纵向变化(资源)
- 进行性核上性麻痹的影像生物标志物(资源)
- 区分皮层基底节变性与其他tau病变的 影像 生物标志物(资源)
- 额颞叶痴呆的神经影像学与临床试验(资源)
- MRI测量进展性核上性麻痹临床试验中的疾病进展(创新报告)
- MRI与皮层基底节变性(创新展示)
- 额颞叶痴呆(FTD)与MRI脑萎缩(创新展示)
- 扩散MRI与额颞叶痴呆(FTD)(创新展示)
研究表明, tau蛋白与β-淀粉样蛋白相比,对 大脑萎缩 的影响 有何不同?
Biospective的研究结果表明,tau病理与脑萎缩和葡萄糖代谢减弱的关系比β-淀粉样蛋白更为密切,且这一关联与APOE ε4基因型无关。这些发现支持了以tau为中心的神经退行性疾病观点。我们最近在 《 阿尔茨海默病与痴呆 》 杂志上发表了一篇期刊文章 :
我们还就该主题发表了创新报告 :
- tau相关萎缩与β-淀粉样蛋白及APOE ε4 无关
- 轻度认知障碍中脑葡萄糖代谢降低由 tau 驱动
相关内容
关于阿尔茨海默病与tau病变的最新研究进展,以及在动物模型中评估治疗药物时使用转化生物标志物的最佳实践。
阿尔茨海默病中的星形细胞形态
星形胶质细胞形态学分析概述及其在神经退行性疾病研究和药物研发中的应用。