What is the significance of TMEM119 in microglia?
Transmembrane protein 119 (TMEM119) is a type I transmembrane protein recognized as a marker of resting homeostatic microglia in the central nervous system (CNS). Microglia act as the primary immune cells of the CNS and play a crucial role in maintaining neuronal health and homeostasis. In addition to its role in the brain, TMEM119 is also involved in the differentiation and proliferation of osteoblasts, as well as bone mineralization.
In 2016, two research groups independently identified TMEM119 as a key marker for microglia:
- The group of Bennet et al. identified TMEM119 as a microglia-specific cell-surface protein of unknown function through in situ hybridization (Bennett, 2016).
- They also found that TMEM119 served as a specific and reliable marker for microglia in both mouse and human CNS, and it was not expressed in macrophages, other immune cells, or neurons.
- Using an extracellular domain antibody, they developed a method to isolate highly pure non-activated microglia cells from the mouse brain.
- The group of Satoh et al. identified that both TMEM119 and Iba1 were expressed in nearly all microglia through a comparative analysis of five datasets of mouse microglia transcriptomes (Satoh, 2016). Their findings were the following:
- TMEM119 was present in Iba1+/CD68+ microglia in post-mortem human brains, regardless of Alzheimer's disease (AD) status, although results varied for reactive microglia.
- TMEM119 was not detected in infiltrating Iba1+/CD68+ macrophages found in active demyelinating lesions of multiple sclerosis (MS) or in necrotic lesions from cerebral infarctions.
- They concluded that TMEM119 is a reliable marker for differentiating resident microglia from infiltrating blood-derived macrophages in the human brain.
Before their publications, distinguishing resident microglia from circulating macrophages in the human brain represented a challenge. The research of Bennet et al. and Satoh et al. has allowed others to assess the role of microglia in the progression of neurodegenerative and neuroinflammatory diseases (Bennett, 2016; Satoh, 2016).
Other complementary markers are crucial for studying microglia:
- P2RY12 (P2Y purinergic receptor 12) serves as another specific marker for homeostatic microglia in humans (Bottcher, 2019; Lier, 2021; Hashioka, 2022):
- Its function includes mediating chemotaxis and the extension of microglial processes towards injured areas.
- P2RY12 is also expressed in oligodendrocytes.
- TMEM119-positive cells also express P2RY12, and vice versa.
- Both markers show significant downregulation or are lost in reactive microglia.
- Iba1, CD11b, CX3CR1, and F4/80 are markers expressed in both microglia and macrophages:
- During neuroinflammation, the expression of Iba1 and CD11b is typically stable or upregulated as microglia become activated (Bottcher, 2019; Bohnert, 2020; Lier, 2021; Ruan, 2022).
- Although F4/80 is present in mouse microglia, it is not found in humans. Its expression patterns can vary in reactive microglia (Bottcher, 2019; Boche, 2022).
- CX3CR1 is crucial for neuron-microglia communication, with stable expression in disease-associated microglia (DAM) (Bennett, 2016; Boche, 2022).
- Refer to our Resource Microglia-Neuron Interactions & Neurodegenerative Diseases for more information about CX3CR1.
Subsequent research has investigated the specificity and has contributed to the understanding of TMEM119 in microglia (Gonzalez Ibanez, 2019; Lier, 2021; Ruan, 2022; Vankriekelsvenne, 2022):
- Under normal physiological conditions, TMEM119 is exclusively expressed by homeostatic microglia. However, its expression is reduced in DAM across various pathological conditions.
- TMEM119 is absent in other brain-resident cells, such as neurons and astrocytes, as well as in peripherally-derived macrophages that may infiltrate the CNS. Nevertheless, other cell types, like follicular dendritic cells in lymphoid organs and brown adipose tissue, can be labelled.
- Together with P2RY12, TMEM119 effectively distinguishes human microglia from circulating myeloid cells found in blood and cerebrospinal fluid (CSF) (Bottcher, 2019).
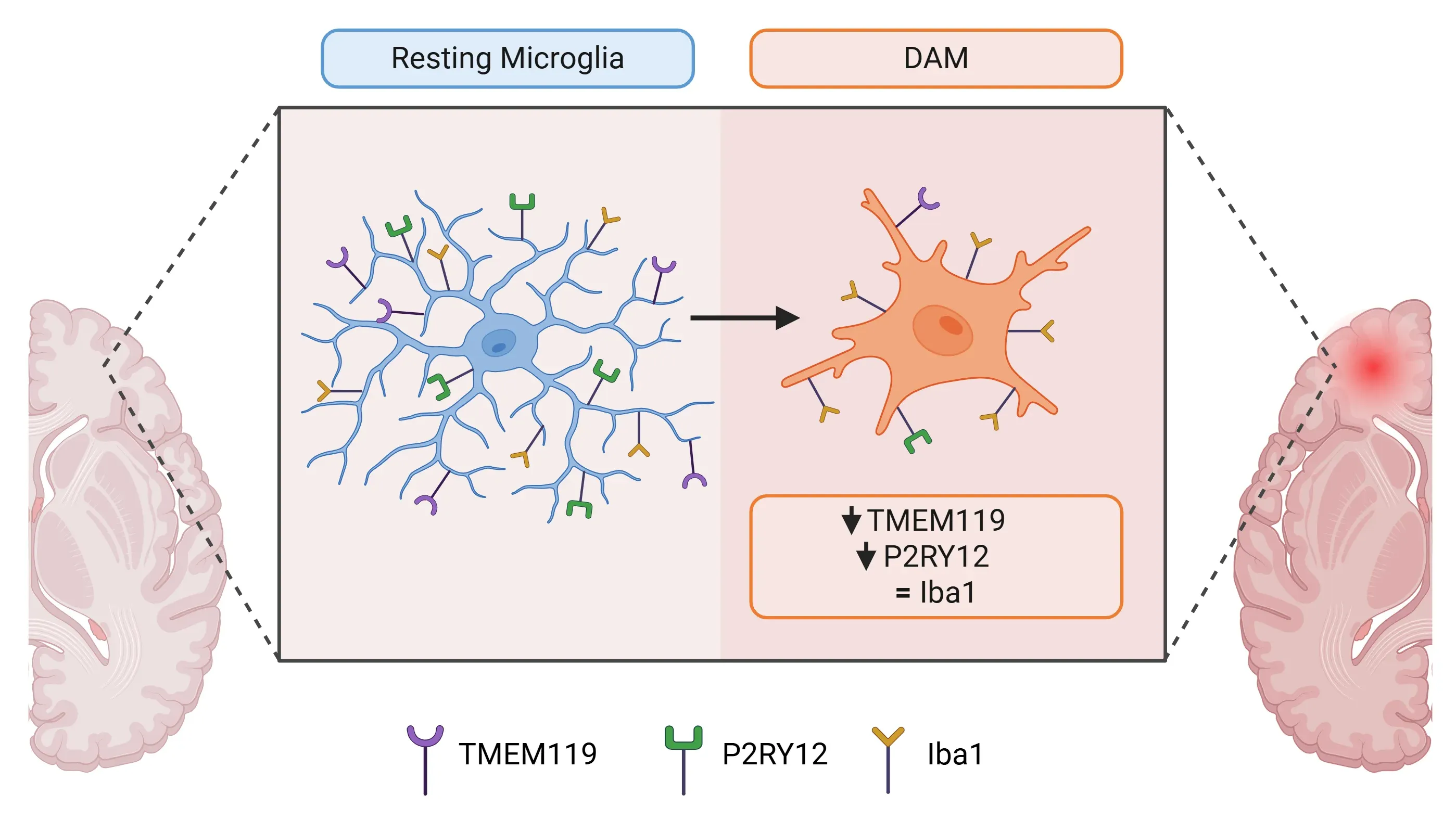
Differences in marker expression between resting microglia and disease-associated microglia (DAM).
What is the role of TMEM119 in Alzheimer’s disease?
In addition to serving as a biomarker for homeostatic microglia, TMEM119 is currently being investigated for its functional implications in AD using mouse models. Here are some insights into its implications:
- Aβ binding and clearance (Liu, 2025):
- TMEM119 directly binds to Aβ oligomers.
- Overexpressing TMEM119 in microglia enhances their phagocytosis ability, promoting the uptake and degradation of Aβ both in vitro and in vivo.
- TMEM119 recruits the low-density lipoprotein receptor 1 (LRP1), which promotes phagocytosis and subsequent lysosomal degradation of Aβ.
- Maintaining microglial homeostasis (Liu, 2025):
- Deficiency in TMEM119 accelerates the transition from homeostatic microglia to DAM.
- Increased Aβ load reduces the expression of TMEM119 in microglia, correlating with disease progression in mice.
- Therapeutic potential (Liu, 2025):
- Enhancing TMEM119 expression using Kartogenin and SRI-011381 has been shown to promote Aβ clearance and improve cognitive function in mouse models of AD.
For more information about the role of Microglia in AD, refer to our Resources APOE4, Microglia & Alzheimer’s Disease and Microglia, Astrocytes & Tau in Neurodegenerative Diseases.
Expression patterns of TMEM119 in AD
The study of TMEM119 expression in the context of AD is complex. TMEM119 expression is often found to be decreased in response to various neurological conditions (Masuda, 2019; Kenkhuis, 2022; Mercurio, 2022; Liu, 2025). In AD, microglia associated with amyloid-beta (Aβ) plaques transition from a homeostatic state to DAM or microglial neurodegenerative phenotype. In this reactive state, the expression of the homeostatic markers, such as TMEM119 and P2RY12, is significantly downregulated, complicating the understanding of TMEM119's role in AD (Hashioka, 2022). Specifically, the following observations have been made:
- There is a significant decrease in TMEM119 expression in microglia within 25 μm of an Aβ plaque, while microglia farther away (≥50 μm) rarely show reduced expression (Liu, 2025). See our Resource Amyloid-β & Inflammatory Microenvironment in Alzheimer's Mice for more information on microenvironment analysis.
- In human patients with AD, there is an overall significant loss of TMEM119-positive microglial phenotypes, regardless of their proximity to Aβ plaques (Kenkhuis, 2022).
- Conflicting results have been reported between tissue and cell analyses in human AD brains (Satoh, 2016; Kenkhuis, 2022):
- Tissue analyses show increased TMEM119 mRNA expression.
- Isolated reactive microglia show reduced expression of TMEM119.
- A loss of TMEM119 expression, alongside an increase in TREM2, is a characteristic feature of the microglial response to Aβ plaques.
- For more information, refer to our Resource TREM2, Microglia and Neuroinflammation.
The role of TMEM119 in microglia in the context of AD is currently under investigation. It remains crucial to differentiate microglia from DAM and other myeloid cells to enhance the understanding of AD development and to create targeted treatments (Hashioka, 2022).
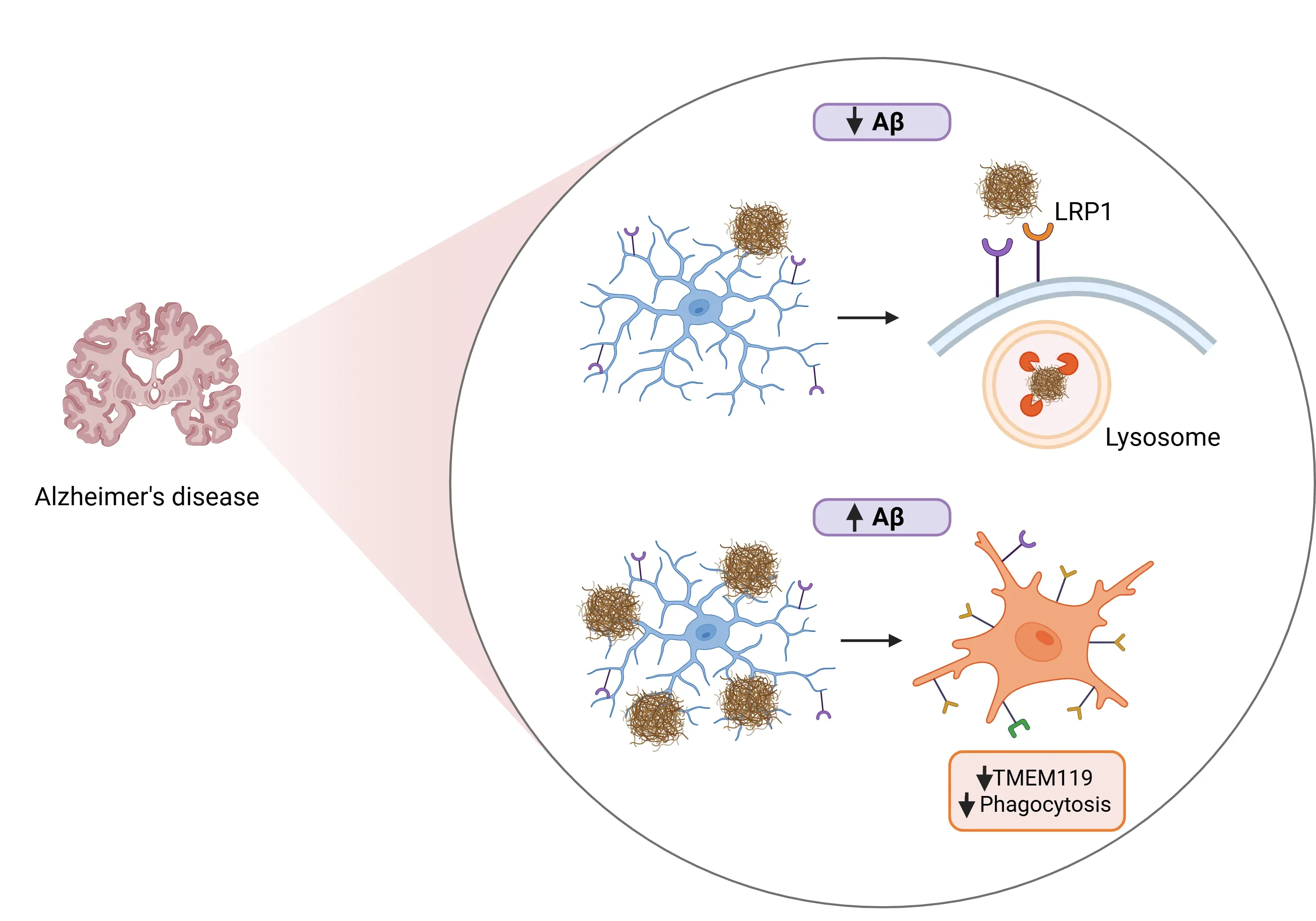
The role of TMEM119 in Alzheimer's disease (AD) under conditions of low versus high amyloid-beta (Aβ) levels.
What is the role of microglial TMEM119 in other pathological conditions?
The role of microglial TMEM119 in pathological conditions beyond AD is primarily as a specific marker for microglia. In conditions where the blood-brain barrier (BBB) is compromised, TMEM119 is reliably expressed by microglia but is absent from infiltrating blood-borne macrophages (Kempuraj, 2024). While its specific functions remain unclear, TMEM119 expression can be both upregulated and downregulated under different pathological conditions:
- Neurodegenerative diseases
- Multiple Sclerosis (MS):
- TMEM119 immunoreactivity is significantly decreased or absent in the center of active white matter lesions (WMLs) but remains unchanged in gray matter lesions. The downregulation of WMLs is correlated with the presence of lymphocytes and inflammatory cytokines, like IFNγ and IL-4 (van Wageningen, 2019).
- In active demyelinating lesions, infiltrating macrophages express Iba1 but are negative for TMEM119 (Satoh, 2016).
- Experimental autoimmune encephalomyelitis (EAE) models:
- TMEM119 expression is significantly decreased in parallel with increased microglial activation (Vankriekelsvenne, 2022).
- Multiple Sclerosis (MS):
- Traumatic brain injury (TBI)
- The number of TMEM119-positive cells is highest in acute TBI cases, returning to control levels in delayed cases (Bohnert, 2020).
- Conflicting findings have been reported (Mercurio, 2022):
- Upregulation of the TMEM119 gene in brain tissue following injury.
- Reduced mRNA and protein levels in isolated reactive microglia.
- Stroke
- TMEM119 immunofluorescence and immunoreactivity are significantly decreased in microglia in brain regions near the infarct, including the ischemic core and penumbra (Young, 2021).
- The authors reported that due to this downregulation in ramified microglia, TMEM119 is not a stable marker in preclinical stroke models, which limits its use in differentiating microglia from infiltrating macrophages in this context (Young, 2021).
- Brain Metastasis
- Cancer
- TMEM119 may exhibit pro-tumorigenic functions, with its expression increased in various cancers, including osteosarcoma (Jiang, 2017), ovarian cancer (Sun, 2021), and breast cancer (Yang, 2021).
- Its upregulation is consistently associated with tumor progression, metastasis, and poor patient survival.
In conclusion, while TMEM119 can be used as a marker for microglia, there are significant limitations to consider. First, not all microglia are labelled by TMEM119 (e.g. DAM), which limits the study of microglia in various neurological conditions. Additionally, TMEM119 does not exclusively label microglia. For instance, it can also label follicular dendritic cells in lymphoid organs and brown adipose tissue. Given this complexity, it is beneficial to use TMEM119 in combination with other markers, such as Iba1 and P2RY12, to more accurately characterize the diverse phenotypes of microglia in both health and disease.
Our team would be happy to answer any questions or provide specific information about the neurodegenerative disease models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
TREM2, Microglia and Neuroinflammation
An overview of TREM2, its role in microglia, links to neurodegenerative diseases, and potential treatment implications.
APOE4, Microglia & Alzheimer’s Disease
An overview of how ApoE4 influences microglial activity in Alzheimer's disease and the development of targeted therapeutics.
Microglia, Astrocytes & Tau in Neurodegenerative Diseases
How glial-driven neuroinflammation fuels tau aggregation, propagation, and neuronal loss in Alzheimer’s disease and other tauopathies.
Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease
How α-synuclein influences microglia and astrocytes in Parkinson’s disease and other synucleinopathies.
Microglia Morphology in ALS, Alzheimer's Disease & Parkinson's Disease
An overview of microglial morphological analysis and the applications to neurodegenerative disease research and drug discovery & development.
Microglia-Neuron Interactions & Neurodegenerative Diseases
A concise review of the direct interactions between microglia & neurons, and how these cell-to-cell interactions may be affected in neurodegenerative diseases.
Impaired Microglia Autophagy in Neurodegenerative Diseases
How impaired microglia autophagy contributes to the progression of neurodegenerative diseases.
Microglial Senescence and Neurodegenerative Diseases
An overview of microglial senescence and its role in neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD).