What is microglial senescence?
Microglia are a heterogeneous population of cells within the central nervous system (CNS), serving as the brain’s resident immune cells. They play an essential role in maintaining homeostasis, responding to injury and infection, and clearing cellular debris. Comprising approximately 10-15% of the total brain cell population, microglia are critical for orchestrating neuroinflammatory responses and contributing to processes like myelination (Greenwood, 2021; Malvaso, 2023). Additionally, they are involved in immune surveillance and synaptic remodeling (Ng, 2023). Recent studies suggest that microglia undergo senescence with aging, leading to functional changes not only in normal aging but also in various disorders, including neurodegenerative diseases (Ng, 2023).
Microglial senescence is associated with several hallmark changes:
- Acquisition of a senescent-associated secretory phenotype (SASP)
- Irreversible cell cycle arrest (Greenwood, 2021; Malvaso, 2023)
- Increased secretion of reactive oxygen species (ROS) and proinflammatory cytokines
- Mitochondrial dysfunction
- High levels of iron and ferritin accumulation
- Altered morphology: process de-ramification, abnormal swellings, cytorrhexis, and vacuolization (Malvaso, 2023).
- Reduced phagocytic activity and impaired motility
For an in-depth review of microglial morphological analysis, see: Microglia Morphology in ALS, Alzheimer's Disease & Parkinson's Disease
If the accumulation of senescent microglia exceeds a certain threshold, possibly due to the paracrine spread of senescence, it can accelerate the progression of age-related diseases, creating a feed-forward loop that increases the number of senescent microglia (Chaib, 2022).
Challenges in defining microglial senescence include:
- Lack of consensus on defining characteristics
- Absence of a specific marker
- Overlap of the senescent phenotype with other cellular states, such as the dystrophic state (Ng, 2023)
- Shared traits with disease-associated microglia (DAM), including telomere shortening
- Classification of senescent microglia as a distinct subset of DAM (Hu, 2021; Samuel Olajide, 2024).
Despite these challenges, the role of microglial senescence in aging-related neurodegenerative diseases has become a focus of research, with the potential to be targeted for therapeutic interventions.
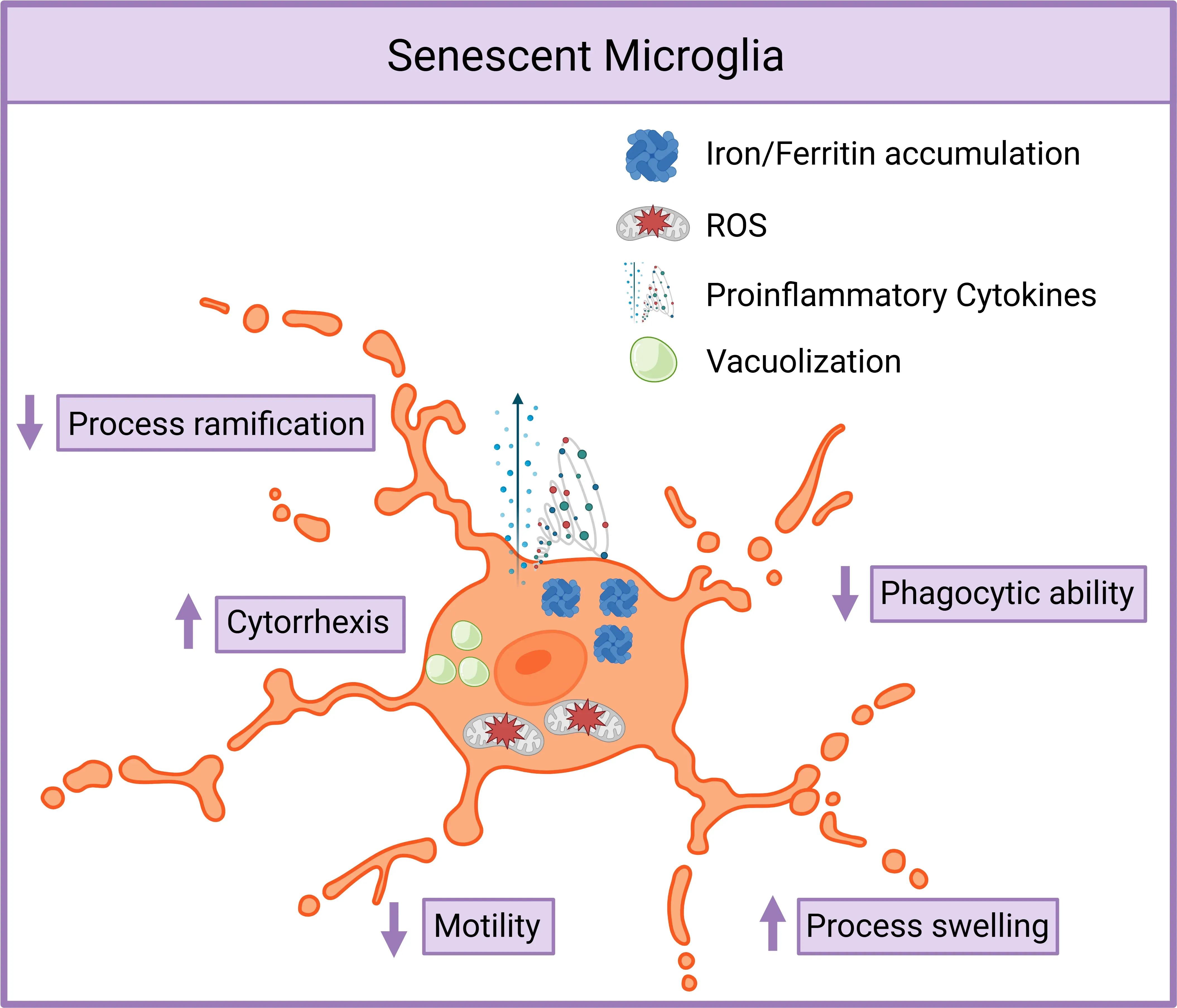
Senescent Microglia Exhibit Dysfunctional and Proinflammatory Phenotype.
Senescent microglia display reduced process ramification and motility, increased swelling, cytorrhexis, vacuolization, and cytoplasmic fragmentation. They accumulate iron, upregulate ferritin, produce more ROS, and secrete elevated proinflammatory cytokines. Phagocytic clearance of debris and toxic proteins is markedly impaired. Figure adapted from Malvaso et al. (Malvaso, 2023) under the Creative Commons Attribution License.
Click to copy link
What role does microglial senescence play in AD and PD?
With the aging of the global population, the incidence of age-related neurodegenerative diseases, including proteinopathies, is expected to increase. Aging serves as a major risk factor for proteinopathies, which are characterized by the accumulation of misfolded proteins like amyloid-beta (Aβ), tau, transactive response DNA binding protein of 43 kDa (TDP-43), and α-synuclein (α-syn). As such, there is a growing demand for targeted therapies aimed at slowing or preventing the progression of these diseases.
Given that senescent microglia build up in the brain and spinal cord during both normal aging and neurodegenerative diseases, they have become a key target for potential therapeutic interventions. Senolytic drugs, which are designed to selectively eliminate senescent cells, aim to mitigate or reverse the effects of aging and inflammation. These drugs have shown promising results in preclinical studies and are currently being tested in early-phase clinical trials to assess their effectiveness in treating age-related conditions, including neurodegenerative diseases.
Alzheimer’s Disease (AD)
AD is a progressive neurodegenerative disorder that primarily leads to cognitive decline, memory impairment, and changes in behavior and mood. The accumulation of Aβ plaques and hyperphosphorylated tau neurofibrillary tangles is central to the pathogenesis of AD. Beyond these hallmark features, microglia play a significant role in the disease process.
A proposed mechanism for disease progression in proteinopathies involves a positive feedback loop between aging, disease, misfolded proteins, and senescent microglia (Lau, 2023):
- Aging promotes the buildup of misfolded proteins
- Misfolded proteins induce microglial senescence
- Senescent microglia have impaired tau clearance and enter cell cycle arrest
- They develop a SASP, releasing inflammatory factors (Karabag, 2023)
- Through paracrine signaling, senescent microglia trigger further senescence in surrounding microglia (Lau, 2023)
- This amplifies protein accumulation, neurodegeneration, and neuroinflammation (Lau, 2023; Miao, 2023).
Parkinson's Disease (PD)
PD, the second most common neurodegenerative disease after AD, is primarily characterized by motor symptoms such as muscle rigidity, bradykinesia, and resting tremor. Despite frequently being overlooked, PD is also associated with non-motor symptoms including mood and affect disorders, such as apathy and depression, as well as cognitive dysfunction and behavioural disturbances. The progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) and the accumulation of Lewy bodies, which contain α-syn aggregates, are defining features of PD.
While microglia are known to contribute to PD progression, the specific role of senescent microglia in PD remains less well understood compared to AD (Rim, 2024). However, several age-related changes suggest a likely connection:
- Microglia accumulation in the SNc increases with age (Shaerzadeh, 2020)
- Older mice show elevated α-syn levels after intra-striatal α-syn injection compared to younger mice (Hong, 2024).
- Aging slows α-syn clearance, likely due to autophagy-lysosome system dysfunction (Hong, 2024).
- Iron accumulation is seen in PD-affected regions like the SNc
- Excessive iron exposure correlates with increased PD risk (Angelova, 2019).
See: Impaired Microglia Autophagy in Neurodegenerative Diseases & Lysosome Dysfunction in Microglia & Astrocytes
In conclusion, although AD and PD have distinct pathological features, they share several common underlying mechanisms, particularly the dysfunction of microglia. In both diseases, microglia contribute to disease progression by promoting inflammation and impairing the clearance of neurotoxic proteins. While significant progress has been made in understanding the role of microglial senescence in neurodegenerative diseases, challenges remain in developing effective therapies. Nonetheless, ongoing research into senolytic treatments holds promise for treating these age-related diseases.
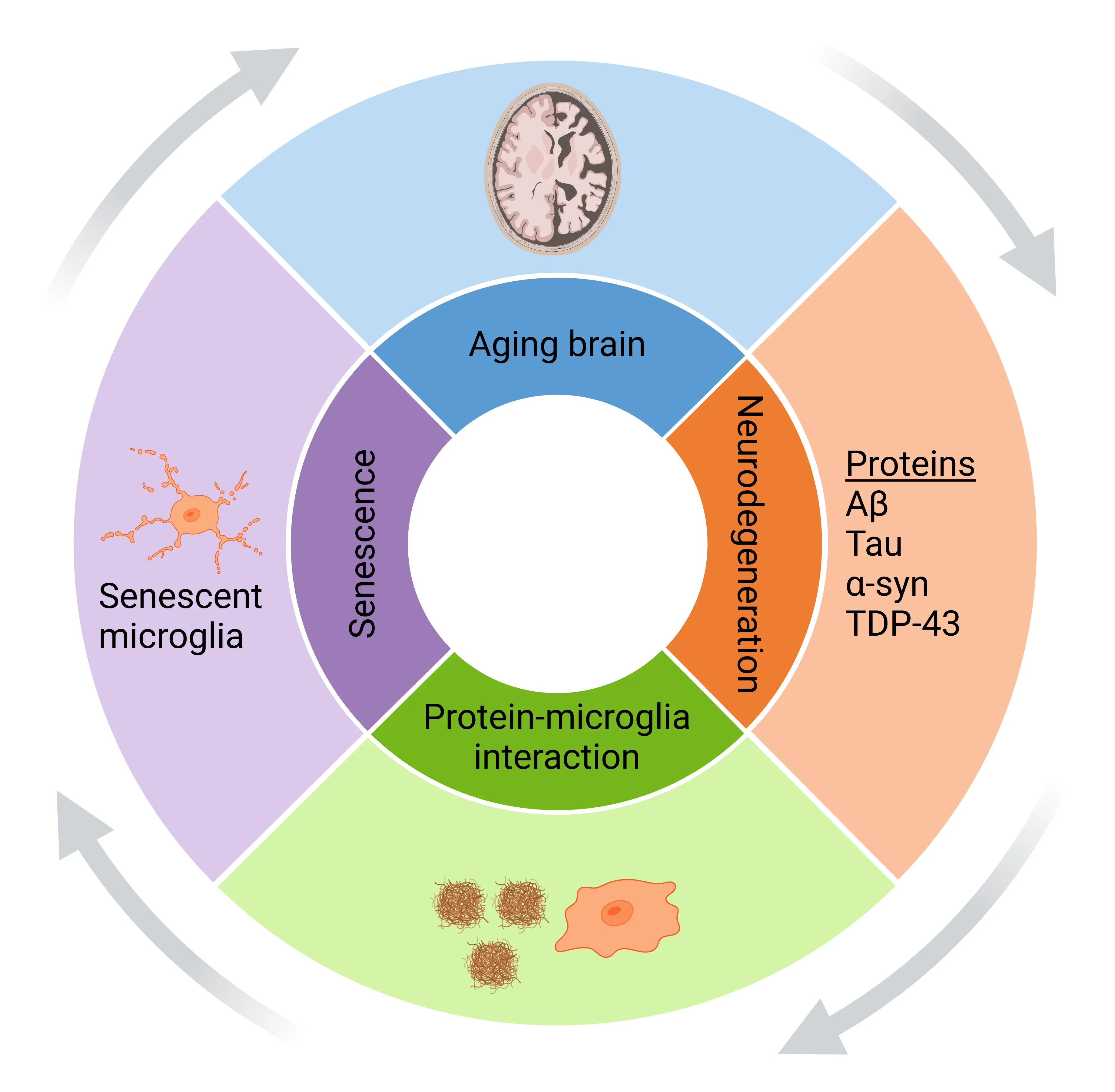
Microglial Senescence Drives Proteinopathy Progression.
With aging and disease, misfolded protein aggregates accumulate and activate microglia, promoting microglial senescence. Senescent microglia exacerbate protein accumulation and neurodegeneration, creating a self-amplifying feedback loop. Figure adapted from Samuel Olajide et al. (Samuel Olajide, 2024) under the Creative Commons Attribution License.
What are the current models and markers used to study microglial senescence?
As research into the role of microglial senescence in neurodegenerative diseases advances, the development of targeted therapeutic strategies has become more urgent. However, the progress of these therapies is hindered by the lack of precise methods for detecting senescent microglia, particularly in vivo. Therefore, the identification of reliable markers for senescent microglia is essential for advancing these therapeutic efforts.
One of the most widely used markers for microglial senescence is senescence-associated β-galactosidase (SA-β-gal) activity, which remains a key indicator. In addition, cyclin-dependent kinase inhibitors such as p21 (also known as WAF1/CIP1) and p16INK4a are frequently used as markers due to their strong association with cell cycle arrest, a hallmark of senescence.
Key markers and tools used to identify senescent microglia include:
- SA-β-gal activity - a traditional hallmark of senescence
- p21 and p16INK4a - cell cycle inhibitors linked to senescence
- Ferritin accumulation - observed in senescent microglia but less well understood
- Sudan Black B staining - detects lipofuscin, which accumulates in aging cells
- SASP cytokines (e.g. TNF-α, IL-6, IL-1β) - released by senescent microglia, contributing to neuroinflammation
Despite the growing body of evidence linking these markers to microglial senescence, no single definitive marker has been established. As a result, a multi-marker approach, combining several of these indicators, is currently considered the most reliable method for identifying senescent microglia.
Animal models have been instrumental in studying microglial senescence. These include the p16luc (luciferase reporter model), p16 knockout, and microglial-specific p16 knockout mice, all of which have helped to clarify p16INK4a's roe in microglial aging and disease.
Experimental models commonly used in research:
- p16luc reporter mice - visualize p16INK4a expression in vivo
- p16 knockout mice - assess the functional role of p16INK4a
- Microglial-specific p16 knockout mice - isolate the effect of p16INK4a loss specifically in microglia
Research has highlighted the importance of markers like p16INK4a, which has been particularly significant in studies of microglial senescence. For example, research on mice has shown that microglia, especially in white matter, are among the first cells in the central nervous system to undergo senescence during aging (Matsudaira, 2023). These studies have confirmed that microglial senescence occurs in both the brain and spinal cord, with senescence being particularly pronounced in DAM (Matsudaira, 2023).
In models of tauopathies, such as the MAPT P301S (PS19) mouse, senescent microglia that express p16INK4a were found to accumulate over time (Bussian, 2018). Targeting these senescent cells has shown therapeutic potential. The clearance of senescent microglia has been found to prevent tau aggregation and neurofibrillary tangle deposition, reduce neurodegeneration, and help preserve cognitive function. Furthermore, the use of senolytics has been shown to modulate tau aggregation. These findings highlight the potential for targeting senescent microglia as a therapeutic approach for treating tauopathies, underscoring the connection between microglial senescence and the progression of neurodegenerative diseases (Bussian, 2018).
By advancing our ability to identify and target senescent microglia, researchers are paving the way for new therapeutic interventions in neurodegenerative diseases. The continued use of these markers and models is essential for advancing our understanding of microglial senescence and its implications for treating age-related neurological conditions.
Our team would be happy to answer any questions about microglial senescence and neurodegenerative diseases or provide specific information about the AD, ALS, and PD models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on microglial senescence and neurodegenerative diseases and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Microglia, Astrocytes & α-Synuclein in Parkinson’s Disease
How α-synuclein influences microglia and astrocytes in Parkinson’s disease and other synucleinopathies.
TNF-α (TNF-alpha) & Microglia in Neurodegenerative Diseases
An overview of the function of tumor necrosis factor-alpha (TNF-α) in microglia and its contribution to the progression of neurodegeneration.
NLRP3 Inflammasome and Neurodegenerative Diseases
An overview of the NLRP3 inflammasome and its role in neurodegenerative diseases, including Alzheimer's disease, Parkinson’s disease, and ALS.
Microglia Morphology in ALS, Alzheimer's Disease & Parkinson's Disease
An overview of microglial morphological analysis and the applications to neurodegenerative disease research and drug discovery & development.
Lysosome Dysfunction in Microglia & Astrocytes
An overview of lysosomal dysfunction in microglia & astrocytes, and its role in neurodegenerative diseases.