Biospective is a leading CRO specializing in high-throughput neuroscience-focused immunofluorescence services for biotech, pharma, and academic researchers globally. We have unique expertise and a strong track record working with mouse & rat models of neurological and neuromuscular diseases. We leverage our fully integrated platform (multiplex tissue staining → whole‑slide scanning → quantitative image analysis) to provide disease model-driven multiplex IF services with pharma-grade rigor.
Our Immunofluorescence (IF) Staining & Analysis Services
High throughput multiplex IF staining and spatial analysis of tissues from research animal models.
At Biospective, our team members are experts in:
- Multiplex immunofluorescence staining
- Fluorescent whole slide scanning
- Automated quantitative analysis of tissue sections
As a Preclinical Neuroscience Contract Research Organization, Biospective provides Research Services for multiplex immunofluorescence staining and analysis to:
- Biotech Industry
- Pharmaceutical Companies
- Research Institutes & Laboratories
We specialize in tissue staining & analysis of:
- Brains
- Spinal Cords
- Muscles
- Peripheral nerves
- Ganglia
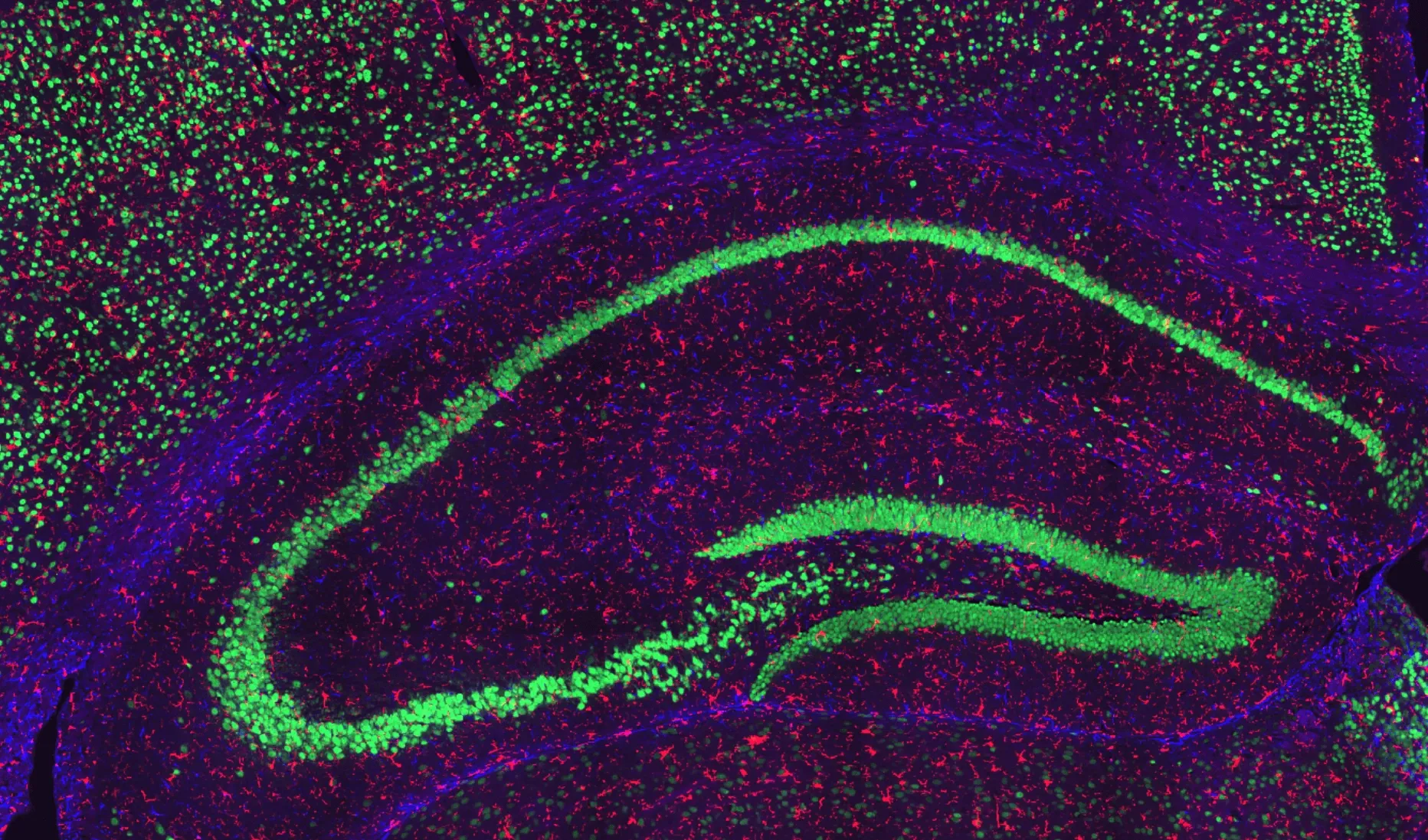
Neuronal and Glial Staining in the Hippocampus of a Wild-Type Mouse.
Representative hippocampal region of a coronal section from a wild-type mouse. NeuN-positive neurons are visualized in green, GFAP-positive astrocytes in blue, and Iba1-positive microglia in red, with nuclei counterstained using DAPI. This staining highlights neuronal populations alongside astrocytic and microglial distributions within the hippocampus.
Our labs leverage state-of-the-art, high-throughput automated immunofluorescence staining instruments and whole slide scanners to maximize quality and minimize turnaround times.
If you are collecting brains, spinal cords, muscles, peripheral nerves, or ganglia as part of your in-house studies or animal model studies performed with another vendor, you can simply ship them to us for staining, slides scanning, and image analysis.
Multiplex Immunofluorescence Studies from Neurological Diseases Models
At Biospective, we routinely perform studies in mouse models of ALS, Alzheimer's Disease & Tauopathies, Parkinson's Disease, and Multiple Sclerosis (MS). Here we provide illustrative examples of our multiplex immunofluorescence staining from these models. You can also learn more about our Multiplex Immunofluorescence (mIF) Services for these models using the provided links.
mIF Staining in ALS (Amyotrophic Lateral Sclerosis) Animal Models
We have extensive experience performing multiplex tissue staining in animal models of ALS.
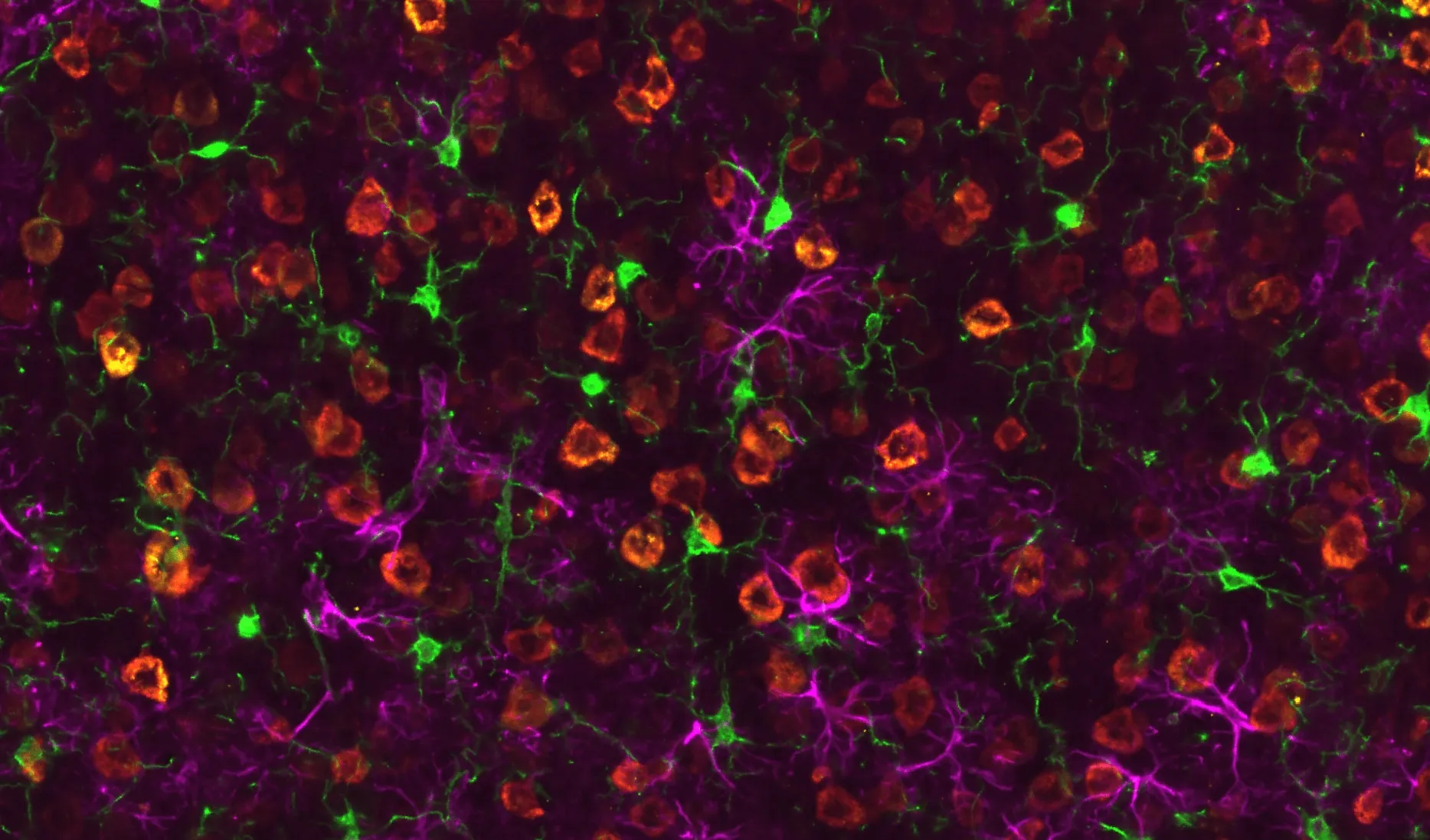
TDP-43 Pathology and Glial Activation in rNLS8 (TDP-43ΔNLS) Mouse Motor Cortex.
Representative motor cortex region of a coronal FFPE section from an rNLS8 mouse. Phosphorylated TDP-43 (pTDP-43) is visualized in yellow, human TDP-43 (hTDP-43) in red, Iba1-positive microglia in green, and GFAP-positive astrocytes in purple. Activated microglia extend processes that contact neuronal soma and regions of TDP-43 accumulation, while astrocytes exhibit reactive GFAP upregulation. This multiplex staining highlights pathogenic TDP-43 aggregation alongside microglial and astrocytic responses characteristic of the rNLS8 ALS model.
In these ALS models, we routinely stain brains and spinal cords for:
- Human TDP-43
- Phosphorylated TDP-43 (pTDP-43)
- GFAP (astrocytes)
- Iba1 (microglia)
- ATP5A (mitochondria)
- Spinal motor neurons (ChAT)
We also stain muscles for neuromuscular junctions (NMJs) using:
- α-bungarotoxin
- SV2A
- β-III-tubulin
Our team has developed robust image analyses for ALS models:
- Please see our Interactive Presentation – Multiplex Immunofluorescence of Brain Sections from the “Low Dox” TDP-43ΔNLS Mouse Model of ALS.
- Visit our "Image Interactive" – Neuromuscular Junction (NMJ) Denervation in the TDP-43ΔNLS (rNLS8) Mouse Model of ALS.
If you have brains, spinal cords, muscle, or other tissue from TDP-43 models and/or other models of ALS (e.g. C9orf72, SOD1, PFN1), we will be happy to work with you.
We can also complement IHC staining by fluid biomarkers from blood and CSF, such as:
- Neurofilament Light Chain (NF-L)
- TDP-43
- GFAP
- Cytokines (e.g. IL-1β, TNF-α)
- Chemokines
- PSD-95
You can learn more about these biomarkers on our Fluid & Cell Biomarkers Services page.
mIF Staining in Alzheimer's Disease & Tauopathies Animal Models
We have validated staining & analysis protocols for amyloid-beta and tau markers, as well as neuroinflammation and neurodegeneration.
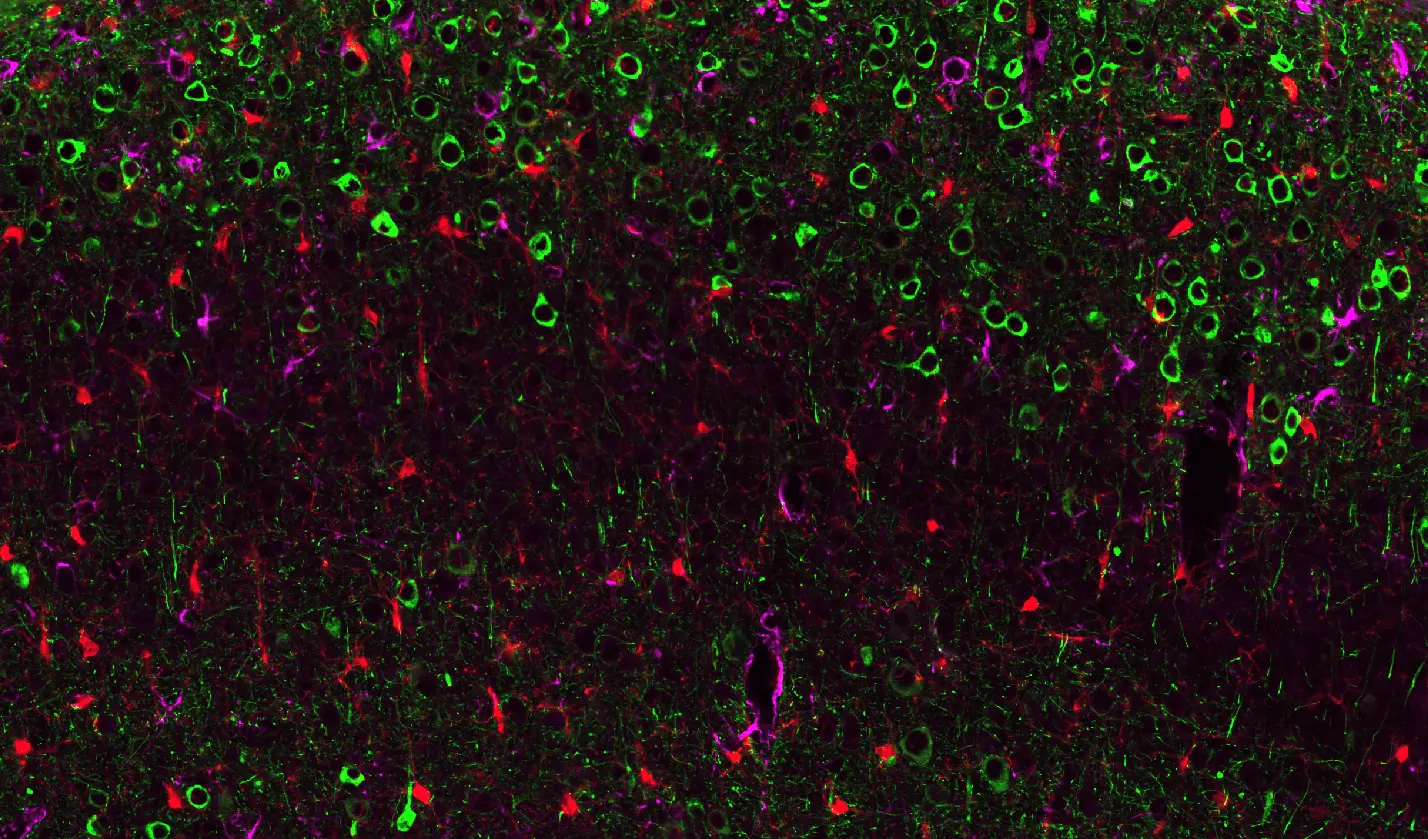
Pathological Tau Aggregates in WT Mice Injected with AAV-hTau.
Representative piriform cortex region of a coronal section from a wild-type mouse brain injected with AAV-hTau. Phosphorylated Tau (AT8) is visualized in green, Iba1-positive microglia in red, and GFAP-positive astrocytes in purple, with nuclei counterstained using DAPI. Activated microglia extend processes toward Tau-positive neurons, while astrocytes exhibit reactive GFAP upregulation.
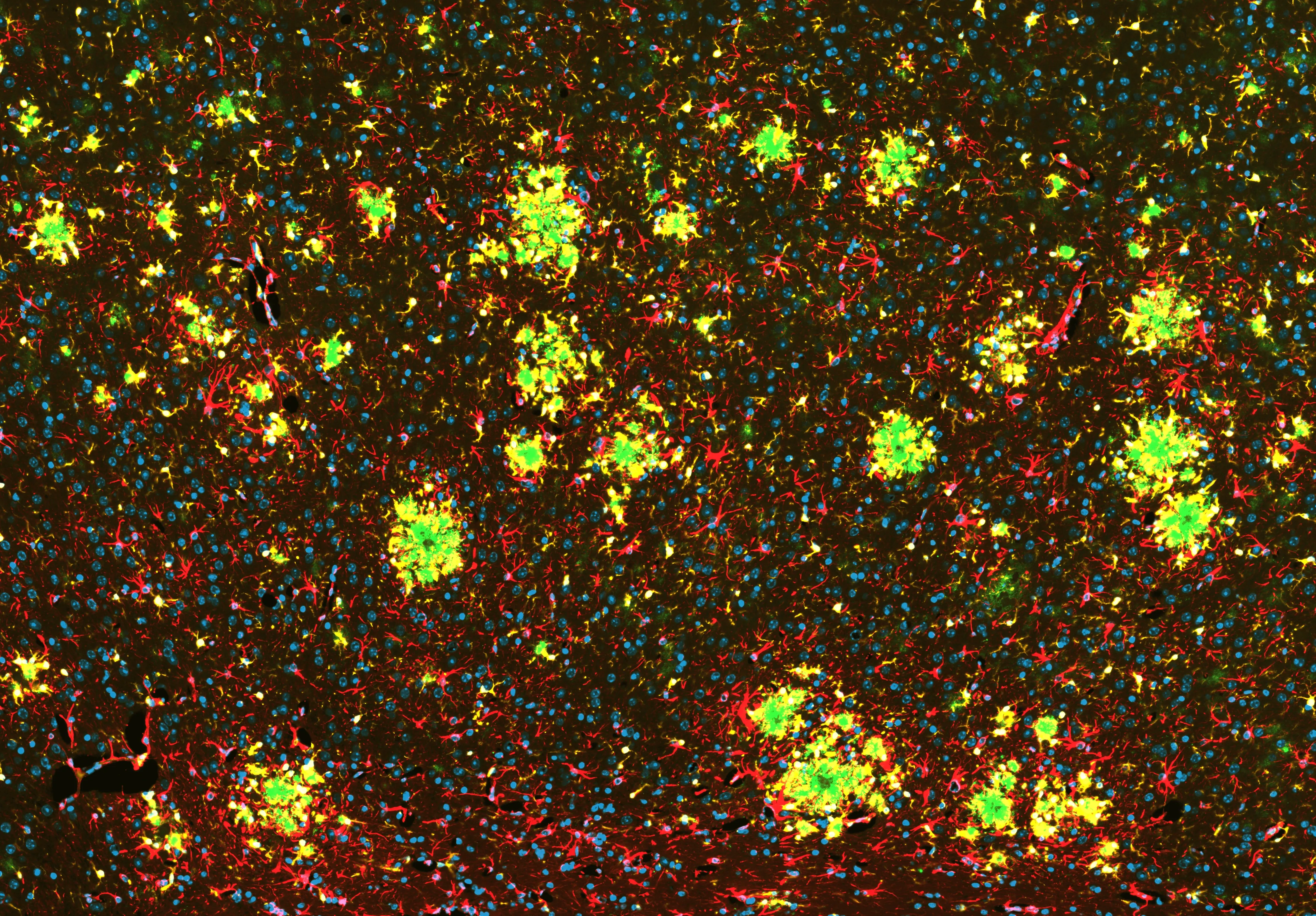
Amyloid-β Pathology and Glial Activation in the APP/PS1 Mouse Brain
Representative coronal section of an APP/PS1 transgenic mouse brain. OC antibody-positive Aβ fibrils are visualized in green, GFAP-positive astrocytes in red, and Iba1-positive microglia in yellow, with nuclei counterstained using DAPI (blue). Activated microglia extend processes toward amyloid deposits, while astrocytes exhibit reactive GFAP upregulation. This multiplex staining highlights fibrillar amyloid-β accumulation alongside coordinated microglial and astrocytic responses in regions affected in this APP/PS1 Alzheimer’s disease model.
In these Alzheimer's Disease & Tauopathy models, we routinely stain brains for:
- Amyloid-beta (various Aβ antibodies)
- Phosphorylated Tau (AT8)
- Conformationally-altered Tau (MC1)
- GFAP (astrocytes)
- Iba1 (microglia)
- NeuN (neurons)
- ASC (inflammasome)
Our team has developed robust image analyses for Alzheimer's disease models, including:
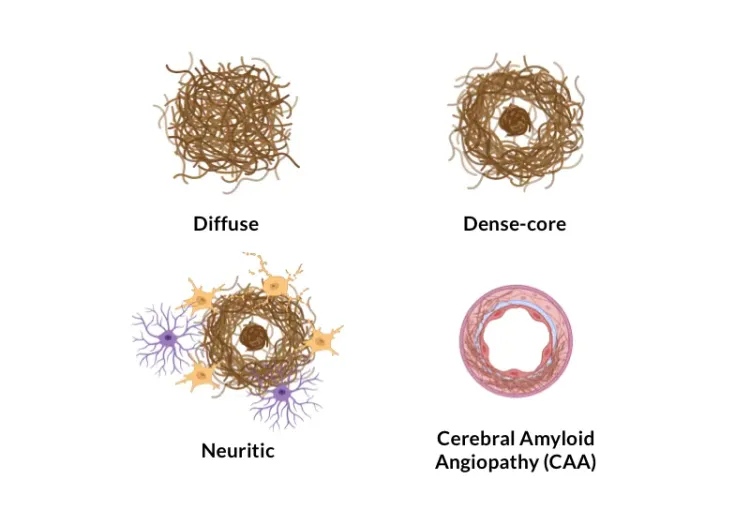
- Amyloid plaque characterization (e.g. size, type [compact, diffuse, fibrillar], count);
- See our Service: Amyloid Plaques Histology Services
- See our Resource: Amyloid-β Plaque Analysis in Alzheimer's Disease
- Inflammatory microenvironment analysis; see our Innovation: Amyloid-β & the Inflammatory Microenvironment in an APP/PS1 Mouse Model of Alzheimer's Disease
- Reactive astrocyte analysis; see our Innovation: Astrocytes & Amyloid-β Mouse Models of Alzheimer's Disease
Please also see our Interactive Presentation – Tau, Rather than Amyloid-β, Drives Neurodegeneration in Alzheimer's Disease (AD) and Mouse Models of AD.
If you have brains or other tissue from APP/PS1 mice and/or other mouse or rat models of Alzheimer's disease or tauopathies (e.g. PS19 mice, 5xFAD mice, APP KI mice or rats, JNPL3 mice, rTg4510 mice), we will be happy to work with you.
We can also complement IHC staining by fluid biomarkers from blood and CSF or brain homogenate supernatants, such as:
- Neurofilament Light Chain (NF-L)
- Aβ40 and Aβ42
- Total tau and phosphorylated tau
- GFAP
- Cytokines (e.g. IL-1β, TNF-α)
- Chemokines
- APOE4
- PSD-95
You can learn more about these biomarkers on our Fluid & Cell Biomarkers Services page.
mIF Staining in Parkinson's Disease Animal Models
We have a wide variety of validated markers for animal models of Parkinson's disease, including α-synuclein and tyrosine hydroxylase (dopaminergic neurons).
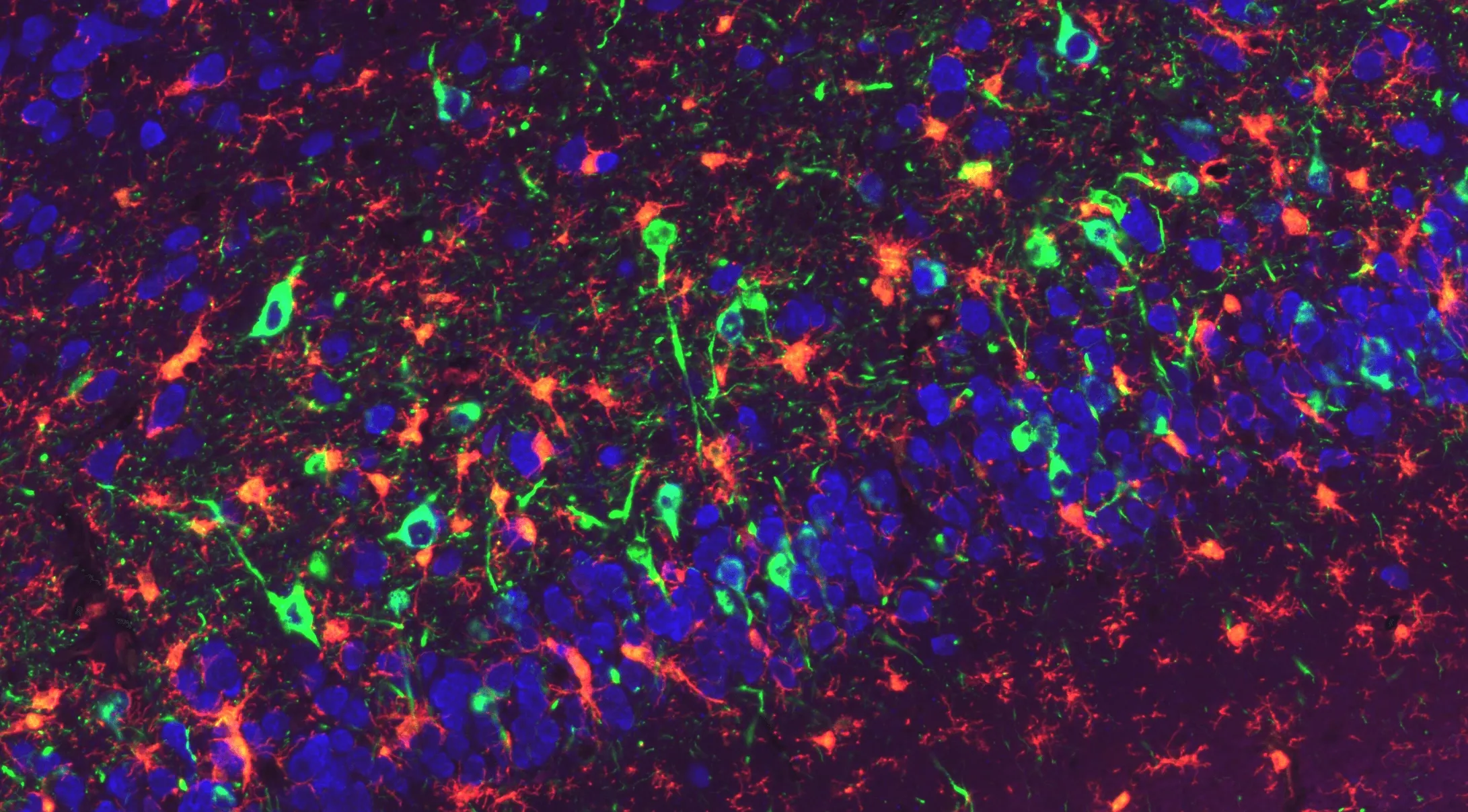
Phosphorylated α-Synuclein Pathology and Microglial Engagement in the Piriform Cortex of M83+/- Mice
Representative piriform cortex region of a coronal FFPE section from an M83 transgenic mouse (overexpressing human A53T α-synuclein) injected with α-synuclein pre-formed fibrils (PFFs). Phosphorylated a-synuclein (pSyn129) is visualized in green, NeuN-positive neuronal nuclei in blue, and Iba1-positive microglia in red. Accumulated pSyn highlights PFF-induced synuclein pathology within neuronal populations, while microglia extend processes toward pSyn-burdened neuronal soma and surrounding neuropil. This multiplex staining delineates the relationship between induced α-synuclein aggregation and microglial activation within the piriform cortex of PFF-injected M83 mice.
In these Parkinson's Disease models, we routinely stain brains for:
- Phosphorylated α-synuclein (pSyn129)
- GFAP (astrocytes)
- Iba1 (microglia)
- NeuN (neurons)
- Tyrosine hydroxylase (dopaminergic neurons)
Our team has developed robust image analyses for Parkinson's disease models, including:
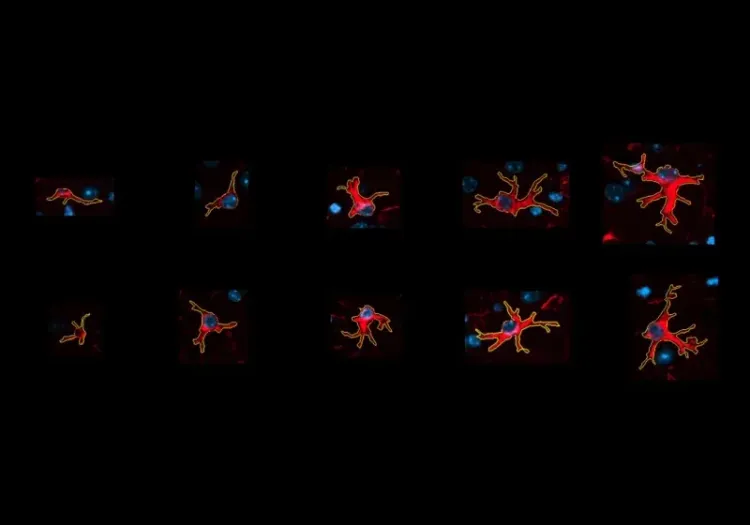
- Activated microglia; see our Innovation: Microglial Activation in an a-Synuclein PFF Mouse Model of Parkinson's Disease
If you have brains from α-synuclein and/or other transgenic, knock-in, knock-out, humanized, or inducible toxin (e.g. MPTP, 6-OHDA, rotenone) mouse or rat models of Parkinson's disease, we will be happy to work with you.
We can also complement IHC staining by fluid biomarkers from blood and CSF or brain homogenate supernatants, such as:
- Neurofilament Light Chain (NF-L) [also see our Resource - Neurofilament Light Chain in Parkinson's Disease Models]
- GFAP
- Cytokines (e.g. IL-1β, TNF-α)
- Chemokines
- PSD-95
You can learn more about these biomarkers on our Fluid & Cell Biomarkers Services page.
mIF Staining in Multiple Sclerosis Animal Models
We have standardized protocols for myelin, peripheral inflammation, and neuroinflammation in animal models of MS.
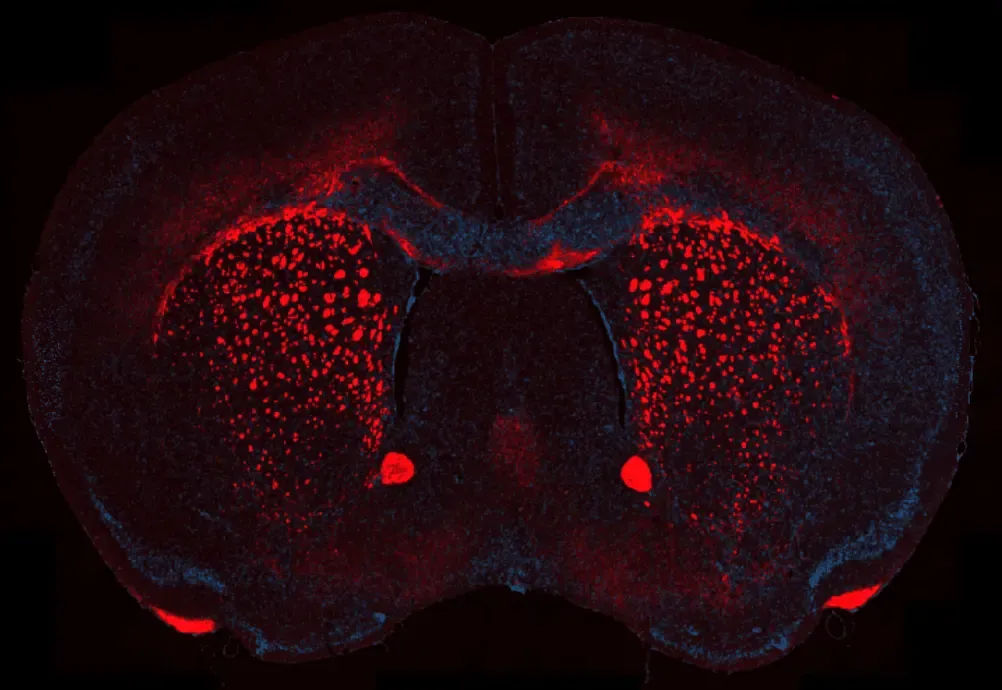
Myelin Loss in the Cuprizone Demyelination Model
Representative corpus callosum region of a coronal FFPE brain section from a mouse with cuprizone-induced demyelination. Myelin basic protein (MBP) is visualized in red, highlighting regions of myelin loss and fragmentation, while nuclei are counterstained with DAPI (blue). This staining delineates the characteristic reduction of compact myelin associated with cuprizone treatment and enables assessment of demyelination severity across affected white matter tracts.
In these Multiple Sclerosis (MS) models, we routinely stain brains and spinal cords for:
- MBP, MOG, or PLP (myelin)
- GFAP (astrocytes)
- Iba1 (microglia & macrophages)
- CD3 (T cells)
- Mature oligodendrocytes
- Oligodendrocyte Precursor Cells (OPC) markers
- Axonal injury markers
Our team has developed robust image analyses for MS models, including:
- Markers of axonal injury & damage; see our Resource: Experimental Autoimmune Encephalomyelitis and Axonal Injury
We can also complement IHC staining by fluid biomarkers from blood and CSF or brain homogenate supernatants, such as:
- Neurofilament Light Chain (NF-L) [also see our Resource - Experimental Autoimmune Encephalomyelitis and Axonal Injury]
- GFAP
- Cytokines (e.g. IL-1β, TNF-α)
- Chemokines
- PSD-95
You can learn more about these biomarkers on our Fluid & Cell Biomarkers Services page.
If you have brains, spinal cords, or other tissue from EAE, cuprizone, and/or other mouse or rat models of Multiple Sclerosis (e.g. LPC), we will be happy to work with you.
What is Multiplex Immunofluorescence (mIF)?
An overview of the mIF technique and its value for spatial analysis in animal models of neurological and neuromuscular diseases.
Multiplex immunofluorescence (mIF) is a powerful technique that enables simultaneous detection of multiple protein targets in the same tissue section using antibodies conjugated to spectrally distinct fluorophores, such as the Alexa Fluor series (AF488, AF555, AF647, AF750). By preserving spatial context, mIF provides detailed maps of cellular co‑expression, subcellular localization, cell–cell interactions, and microenvironment organization. This approach enables direct visualization of interactions between multiple markers in brain, spinal cord, muscle, etc., such as pathogenic protein aggregates, activated glial cells, and neuronal populations, within the same tissue region. In neurodegenerative disease research, mIF is critical for dissecting complex pathology — including co‑aggregation of disease proteins, heterogeneous glial phenotypes, microglial–neuronal contacts/interactions, and neuroinflammatory responses — and supports robust quantitative evaluation of disease progression, spatial biomarker analysis, and therapeutic effects (Hickey, 2021; Park, 2022; Bull, 2024).
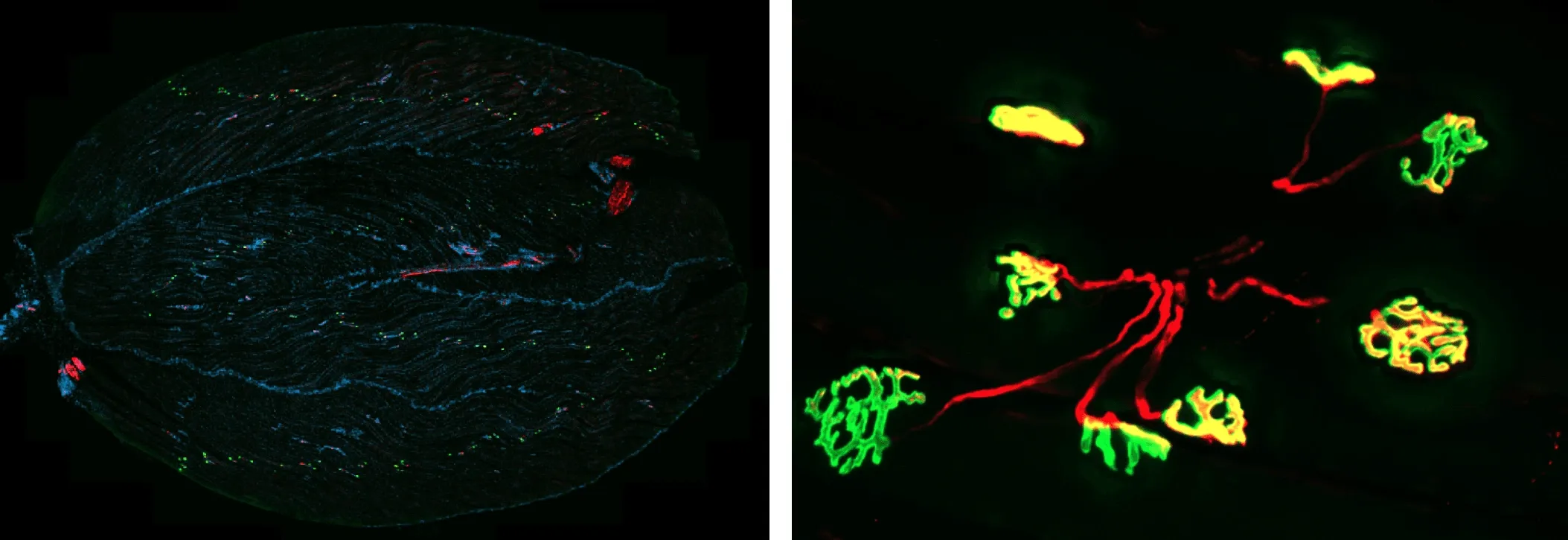
Neuromuscular Junction (NMJ) Visualization in Mouse Gastrocnemius Muscle.
(Left) Representative section of mouse gastrocnemius muscle. α-Bungarotoxin (BTX) labeling of acetylcholine (ACh) receptors is visualized in green, synaptic vesicle protein SV2A and tubulin are visualized in red, and nuclei are counterstained with DAPI (blue). This staining allows visualization of neuromuscular junction architecture, including presynaptic and postsynaptic compartments, within muscle fibers.
(Right) Higher magnification image of NMJ showing partially- and fully-innervated NMJs.
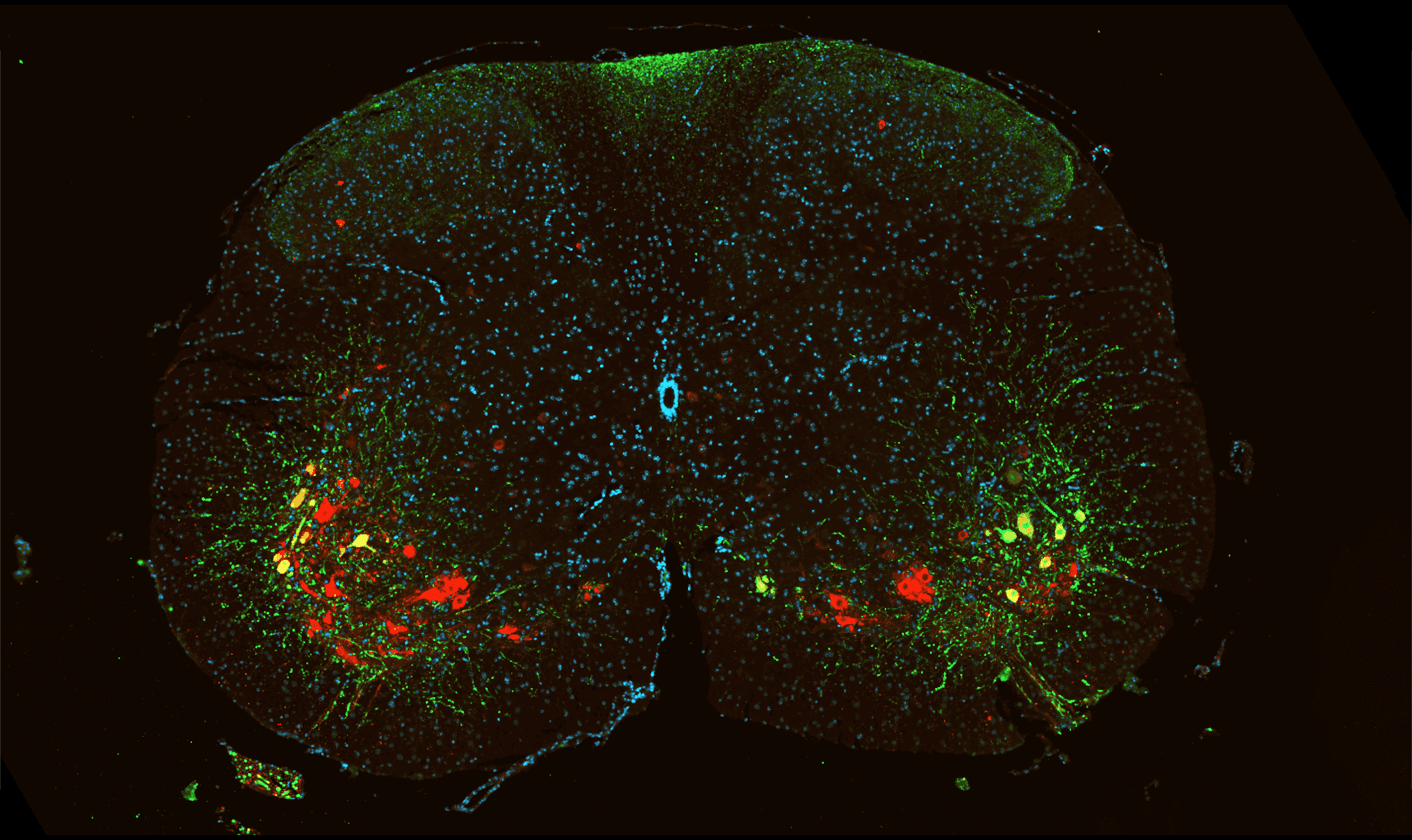
Motor Neuron Visualization in Mouse Spinal Cord.
Representative section of mouse spinal cord. GFP-expressing neurons are visualized in green and ChAT-positive cholinergic neurons are visualized in red. Nuclei are counterstained with DAPI (blue).
Illustration of Biospective's process of collecting tissue from animal models, performing tissue sectioning, multiplex immunofluorescence staining, whole slide scanning, and quantitative image analysis.
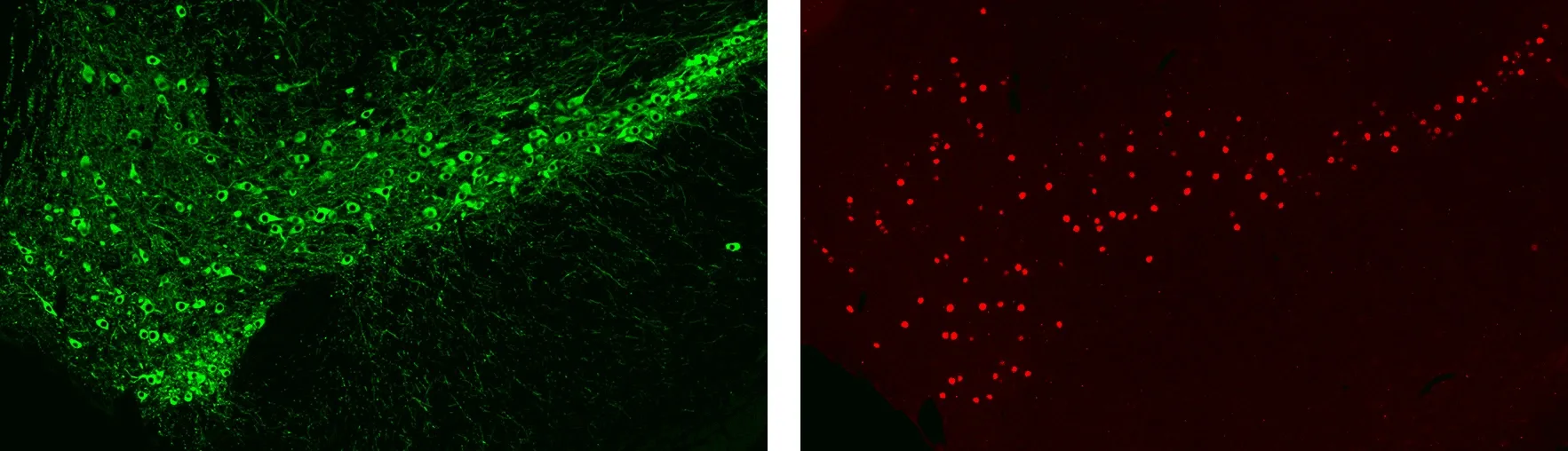
Immunofluorescence (IF) Staining of the Mouse Substantia Nigra for Dopaminergic Cell Bodies & Processes (left) and Neuronal Nuclei (right).
At Biospective, our laboratories utilize multiple automated IHC/IF staining instruments, ensuring:
- High reproducibility
- Low variability
- Rapid turnaround
- Optimal consistency across large cohorts
We maintain well-validated multiplex IF panels for rodent models of neurological diseases and can develop custom staining protocols tailored to your research needs. Our dedicated R&D team of experienced scientists and technicians ensures that every protocol is optimized for reproducibility, sensitivity, and high-quality quantitative analysis.
To learn more about our Multiplex Immunofluorescence Services
Discover more of our Immunofluorescence Staining Services
Related Content
Up-to-date information on our Multiplex Immunofluorescence Services and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Amyloid-β Plaque Analysis in Alzheimer's Disease
Overview of methods to classify & quantify Aβ plaques in brain tissue sections from humans & Alzheimer’s disease animal models (transgenic mice & rats).
Amyloid-β & Inflammatory Microenvironment in Alzheimer's Mice
We have analyzed the complex spatial relationships between β-amyloid plaques, activated & resting microglia, and astrocytes in an APP/PS1 transgenic model.
TDP-43 ΔNLS (rNLS8) Mice for ALS Drug Development
This resource provides information about the use of the ΔNLS (deltaNLS, hTDP-43ΔNLS, hTDP-43DeltaNLS, dNLS, TDP43 NLS, rNLS8) TDP-43 transgenic mouse model of ALS for preclinical therapeutic studies.
A Guide to ALS Models for Drug Discovery
A Resource for the most effective use of research animal models (mouse & rat models) of Amyotrophic Lateral Sclerosis (ALS) for preclinical testing of therapeutics.
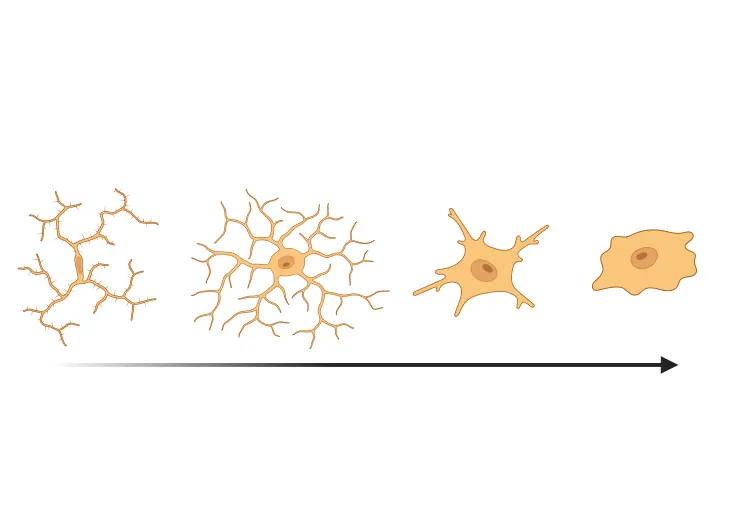
Astrocytes & Amyloid-β Mouse Models of Alzheimer's Disease
Analysis of astrocyte morphology in the amyloid-β plaque microenvironment provides a sensitive measure of disease progression in transgenic mice.
Microglial Activation in an α-Synuclein PFF Mouse Model
We have quantified microglial activation, based on morphology, in an α-synuclein preformed fibril (PFF) seeding & spreading mouse model of Parkinson’s disease.
Astrocyte Morphology in Alzheimer's Disease
An overview of astrocyte morphological analysis and the applications to neurodegenerative disease research and drug discovery & development.
Microglia Morphology in ALS, Alzheimer's Disease & Parkinson's Disease
An overview of microglial morphological analysis and the applications to neurodegenerative disease research and drug discovery & development.
Microglia-Neuron Interactions & Neurodegenerative Diseases
A concise review of the direct interactions between microglia & neurons, and how these cell-to-cell interactions may be affected in neurodegenerative diseases.
Demyelination & Remyelination in the Cuprizone Model
An overview of the methods available to measure myelin and oligodendrocytes in the cuprizone demyelination mouse model of multiple sclerosis (MS).
Experimental Autoimmune Encephalomyelitis (EAE) & Axonal Injury
This resource describes the methods available for measuring axonal damage & axon degeneration, including tissue markers and plasma & CSF neurofilament light chain (NfL; NF-L) levels, in the EAE model of multiple sclerosis (MS).