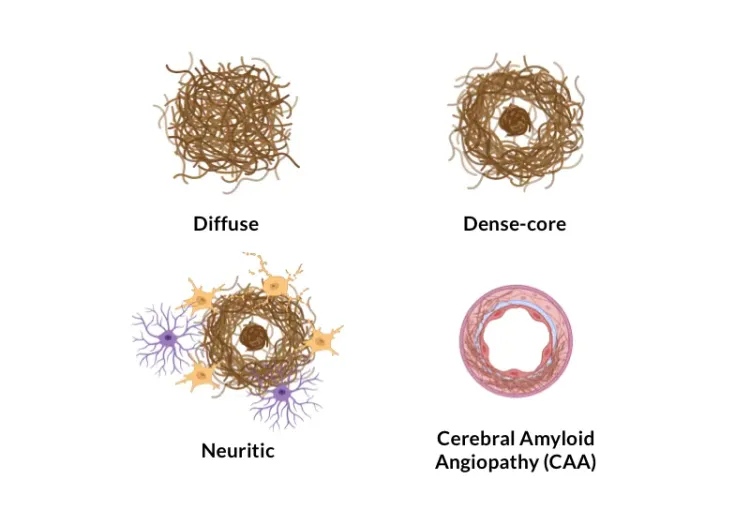
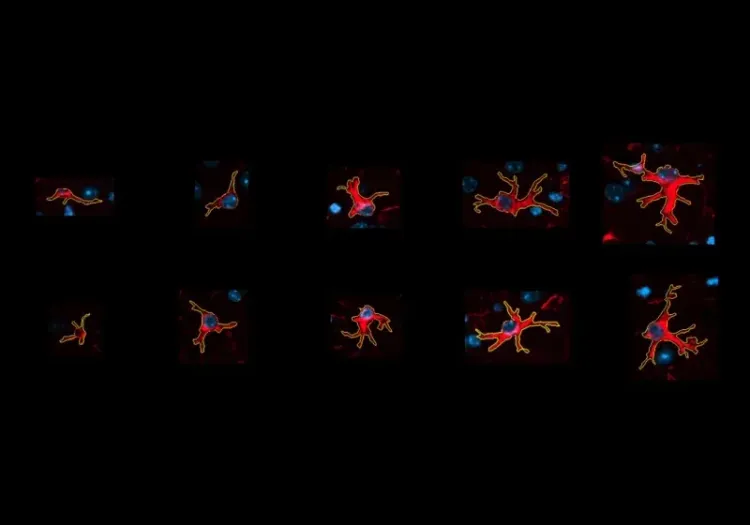
Summary
This Resource provides a comparative, mechanistic overview of the 5xFAD and APP/PS1 transgenic mouse models - two of the most widely used models for studying amyloid-β-driven Alzheimer's disease (AD) pathology. Both models overexpress human APP and PSEN1 with familial AB mutations to accelerate Aβ plaque formation, yet they differ substantially in the timing and regional distribution of pathology, as well as in their neuroinflammatory and cerebrovascular profiles.
The APP/PS1 model shows a regionally concentrated accumulation of Aβ in the cortex and hippocampus, and develops cerebrovascular amyloid angiopathy (CAA) in specific lines, while maintaining relatively stable motor behavior. In contrast, 5xFAD mice exhibit early, progressive and widespread Aβ deposition accompanied by a rapid onset of microgliosis & astrogliosis and behavioral impairments.
This comparison summarizes their genetic design, temporal progression of pathology, inflammatory and vascular phenotypes, and relevance for ARIA studies - along with limitations such as the absence of tauopathy - to support informed model selection for AD therapeutic research.
What are the APP/PS1 and 5xFAD mouse models?
The APP/PS1 and 5xFAD mouse models are widely used double-transgenic Alzheimer’s disease (AD) models, specifically designed to accelerate and intensify amyloid-β (Aβ) pathology. These models overexpress mutated human Amyloid Precursor Protein (APP) and Presenilin 1 (PSEN1) - genes known to cause early-onset familial Alzheimer's disease (FAD) - thereby driving rapid Aβ plaque formation (Sasaguri, 2017; Fang, 2024).
While both models exhibit robust Aβ deposition, they differ markedly in their:
- Genetic composition
- Speed of pathology development
- Aβ plaque characteristics
- Neuroinflammatory profile
- Behavioral deficits
Importantly, tauopathy does not naturally develop in these models (although Biospective has developed an APP/PS1 and human Tau co-pathology model for advanced AD research). These two transgenic mouse models serve as valuable tools for exploring the mechanisms underlying AD and for testing new therapeutic interventions.
Comparative Overview of the APP/PS1 and 5xFAD Mouse Models in Alzheimer's Disease Research
| Category |
APP/PS1 (ARTE10) |
5xFAD |
| Genetics |
|
|
| Disease Progression |
|
|
| Key Features |
|
|
Comparative overview of APP/PS1 and 5xFAD Alzheimer's disease mouse models, highlighting key differences in genetic background, temporal dynamics of amyloid-β deposition, associated neuroinflammatory and neurodegenerative readouts, and research-relevant phenotypic features.
What are the characteristics of both models in relation to amyloid-beta pathology, neuroinflammation, and cerebrovascular changes?
What are differences in Amyloid-β Pathology between APP/PS1 and 5xFAD mice?
APP/PS1 model
- The APP/PS1 model exhibits accelerated and robust Aβ pathology, with deposits appearing as early as 3 to 4 months of age, primarily concentrated in the cerebral cortex and hippocampus (Zhong, 2024).
- Impairments in spatial memory and overall cognitive function are observed around 6 months, although some studies have reported memory impairment as early as 17 weeks (Fang, 2024; Noto, 2025).
- Histological analysis shows the presence of dense and mature senile plaques by 8 months (Shen, 2018).
- Aβ deposition in these mice is associated with a range of downstream effects, including synaptic dysfunction and minor neuronal loss near the amyloid plaques at around 8 to 10 months (Zhong, 2024).
- Overall, motor skills generally remain intact (Webster, 2013).
Explore Spatial Pathology:
For detailed spatial analysis of Aβ and inflammatory cells in the APP/PS1 model, see our Innovation: Amyloid-β & the Inflammatory Microenvironment in an APP/PS1 Mouse Model of Alzheimer’s Disease.
5xFAD model
- In the 5xFAD model, amyloid plaques become easily identifiable throughout the brain starting at 2 months, with their accumulation peaking around 5 months, although the disease may progress over time (Oblak, 2021; Bader, 2023; Padua, 2024).
- Initial extracellular amyloid plaques form in the subiculum of the hippocampus and the frontal cortex, later spreading to other regions of the brain. Increased pro-inflammatory cytokines, such as IL-1β and TNF-α, are also observed (Fang, 2024).
- Impairments in spatial memory begin around 4 to 5 months (Fang, 2024).
- Motor dysfunctions start to develop at approximately 9 months (Zhong, 2024).
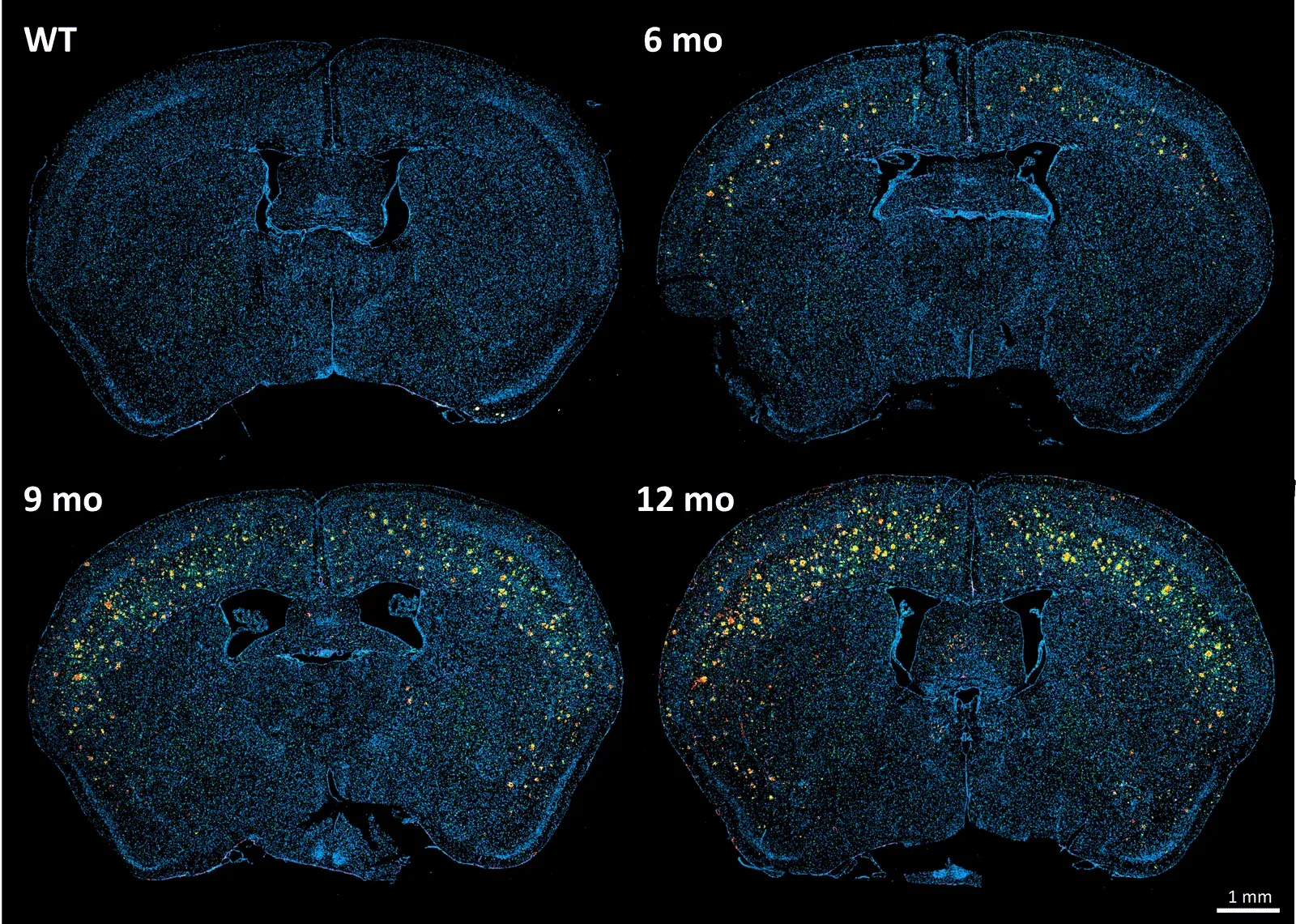
Brain sections of the APP/PS1 (ARTE10) transgenic mouse model and wild-type mice at various time points, stained for Aβ (red), Iba-1 (green), GFAP (violet), and DAPI counterstain (blue).
What are differences in Neuroinflammation between APP/PS1 and 5xFAD mice?
APP/PS1 model
- In this model, there is increased activation of microglia and astrocytes observed as early as 4 months of age (Zhong, 2024; Fang, 2024).
- By 6-7 months, there is a noticeable increase in the number of Iba-1 positive microglial cells, predominantly around the amyloid deposits in the hippocampus (Campos, 2023).
- The accumulation of multivesicular bodies, endosomes, and lysosomes, has been reported (Zhong, 2024).
- Clear neurodegeneration in the striatum is evident by 1 year of age (Fang, 2024).
- The ARTE10 model has been used to target the inflammasome pathway with a novel anti-ASC immunotherapy
5xFAD model
- In the 5xFAD model, reactive microglia and astrocytes can be detected by 2 months (Padua, 2024).
- Dystrophic neurites start to appear by 3 months, leading to observable deficiencies in synaptic transmission by approximately 4 months of age (Padua, 2024).
- By 1 year of age, microgliosis and astrogliosis are evident in both the cortex and hippocampus (Oblak, 2021; Fang, 2024).
- There is a significant loss of synapses around 6 months, leading to a 40% loss of neurons by 1 year, with a substantial decline in neuronal health observed by approximately 9 months (Fang, 2024; Padua, 2024).
- Apathy-like behaviors have been observed to positively correlate with Aβ pathology, particularly the presence of soluble Aβ42 in the prefrontal cortex and hippocampus (Keszycki, 2023).
What are differences in Cerebrovascular Pathology between APP/PS1 and 5xFAD mice?
Cerebrovascular dysfunction, including cerebral amyloid angiopathy (CAA), plays a significant role in the initial pathogenic changes associated with AD.
APP/PS1 model
- While the standard APP/PS1 model shows limited CAA, certain models exhibit CAA (Fang, 2024). The ARTE10 model has extensive CAA by 9 months of age.
- In APP/PS1 mouse models with the Swedish and ΔE9 mutations, the onset of CAA can begin as early as 6 months of age, accompanied by extensive plaque formation and elevated levels of Aβ42 (Noto, 2025).
- An APP/PS1 rat model (TgF344) incorporates the Swedish and ΔE9 mutations with a PrP promoter and develops CAA, amyloid plaques, cognitive impairment, and neurofibrillary tangles (NFT) (Fang, 2024).
- Decreased cerebral blood flow in the left hippocampus, left thalamus, and right cortex has been detected in 8-month-old APP/PS1 mice using arterial spin labeling (ASL) MRI, indicating vascular dysfunction in this AD model (Shen, 2018).
- The deposition of amyloid on the vascular wall disrupts the integrity and morphology of the arterial basement membrane in APP/PS1 mouse models. As CAA progresses, there is a decrease in vascular smooth muscle cell coverage and impairment of Aβ clearance mechanisms, including intramural periarterial drainage and perivascular CSF influx (Kim, 2020).
5xFAD model
- Overall, this model is generally regarded as having limited cerebrovascular dysfunction (Fang, 2024).
- However, it has been reported that CAA can be observed as early as 3 months of age. Impaired neurovascular coupling is observed at 12 months, although cerebral blood flow remains unchanged (Fang, 2024).
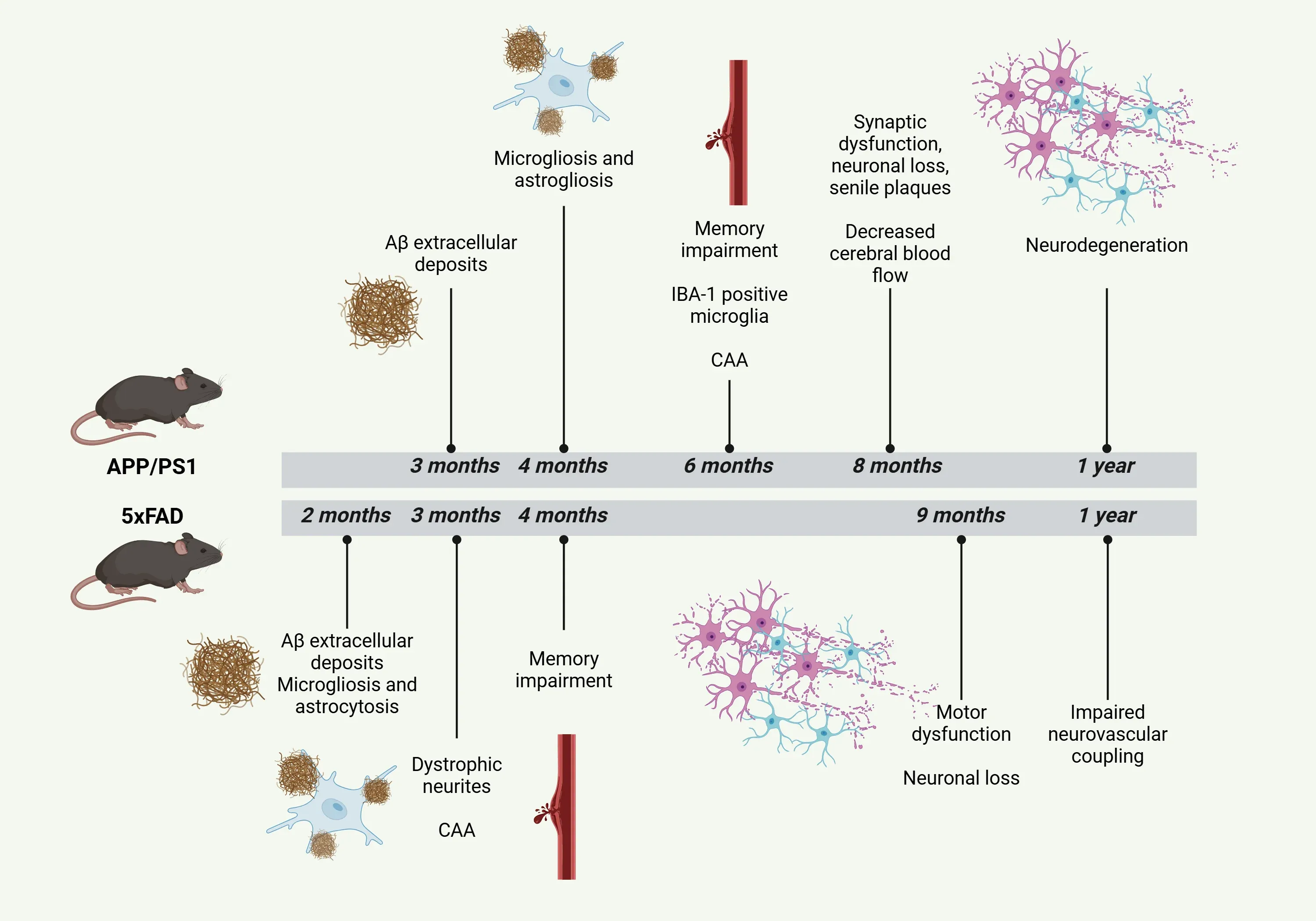
Timeline of the pathological characteristics of APP/PS1 and 5xFAD mouse models for AD.
What are differences in Amyloid-Related Imaging Abnormalities (ARIA) Modeling between APP/PS1 and 5xFAD mice?
ARIA, which include edema/effusion (ARIA-E) and hemosiderosis/microhemorrhage (ARIA-H), are adverse events associated with anti-Aβ antibody treatments (Hampel, 2023; Grenon, 2024).
- These abnormalities are more common in individuals who are carriers of the APOE4 allele.
- Most cases of ARIA are asymptomatic. However, ARIA can lead to symptoms such as headache, confusion, nausea, vomiting, and dysfunction in vision and gait.
- MRI monitoring is crucial for identifying and managing ARIA.
- Typically, ARIA resolves within 3 to 4 months through the adjustment or discontinuation of the medication.
- Spontaneous ARIA can also occur in humans who have CAA.
Therefore, animal models are essential for a deeper understanding of the pathophysiology of ARIA.
APP/PS1 model
- Given the extensive CAA and vascular pathology in the ARTE10 model (at ~9 months of age), there is significant potential to utilize this model to study ARIA in pharmacological studies of anti-amyloid therapies (e.g. immunotherapy).
- Recent studies have employed the APP/PS1 model carrying the APOE4 gene to explore the vascular side effects of anti-amyloid therapy in AD (Grenon, 2024).
- The APP/PS1-21 model has been used to evaluate microhemorrhages when treated with the murine precursor mIgG2a antibody representing Bapineuzumab, 3D6, and ABBV‑916, the murine precursor of a clinical stage human IgG1 monoclonal antibody which binds to N-terminal truncated, pyroglutamate-modified at amino acid position 3, Aβ (AβpE3) under development by AbbVie (Liao, 2025).
5xFAD model
- This model is not commonly used for ARIA studies in the literature. However, a recent investigation examined the therapeutic effects of anti-APOE (HAE-4) in 5xFAD mice expressing the APOE4 allele (Xiong, 2021). As such, the 5xFAD x APOE4 double transgenic model can be used to study ARIA events.
Are Neurofibrillary Tangles (NFTs) present in APP/PS1 and 5xFAD mice?
Both models are characterized by the absence of neurofilbrillary tangles (NFTs), which are crucial pathological features of AD. To overcome this limitation, Biospective has developed and characterized and APP/PS1/hTau amyloid-beta and tau co-pathology mouse model, see: Amyloid-Beta & Tau Co-Pathology Mouse Model (APP/PS1/hTau).
How does the APP/PS1 model compare to the 5xFAD model?
| Pathologic Feature |
APP/PS1 |
5xFAD |
Advantage of using APP/PS1 model |
| Amyloid pathology |
|
|
|
| Neuroinflammation |
|
|
|
| Cerebrovascular pathology |
|
|
|
| ARIA modeling |
|
|
|
This table provides a side-by-side comparison of APP/PS1 and 5xFAD models across four key pathologic features: amyloid-β pathology, neuroinflammation, cerebrovascular pathology, and ARIA modeling. It also highlights the advantages of using APP/PS1 model in each of the areas.
Our team would be happy to answer any questions about the 5xFAD & APP/PS1 Mice or provide specific information about the models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Amyloid-β & Inflammatory Microenvironment in Alzheimer's Mice
We have analyzed the complex spatial relationships between β-amyloid plaques, activated & resting microglia, and astrocytes in an APP/PS1 transgenic model.
Amyloid-β Plaque Analysis in Alzheimer's Disease
Overview of methods to classify & quantify Aβ plaques in brain tissue sections from humans & Alzheimer’s disease animal models (transgenic mice & rats).
Astrocytes & Amyloid-β Mouse Models of Alzheimer's Disease
Analysis of astrocyte morphology in the amyloid-β plaque microenvironment provides a sensitive measure of disease progression in transgenic mice.