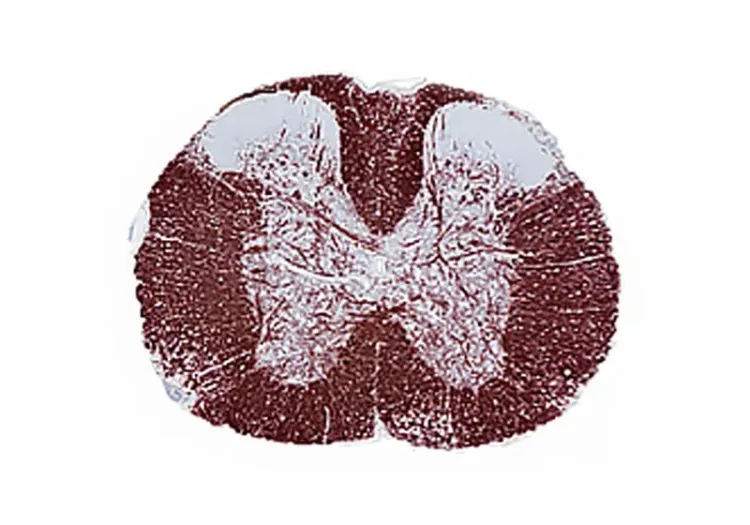
What is EAE?
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune disease primarily driven by T-helper cells that invade the central nervous system (CNS). This condition leads to inflammation, demyelination, and neurodegeneration in the CNS, along with an early disruption of the blood-brain barrier (BBB) (Robinson, 2014; Terry, 2016; Dhaiban, 2021).
EAE serves as one of the key animal models for investigating multiple sclerosis (MS). Much of our understanding of MS stems from studies involving EAE, and the treatments explored in this model are often translated to human applications. Notably, EAE exhibits certain similarities with the human progression of MS, manifesting histopathological lesions characterized by neuroinflammation, gliosis, demyelination, and axonal injury (Robinson, 2014; Terry, 2016; Hasselmann, 2017).
Refer to our Resources: Axonal Injury & Experimental Autoimmune Encephalomyelitis & Demyelination & Remyelination in the Cuprizone Model
In addition to EAE, researchers employ various other models to study MS, such as demyelination models triggered by toxins like cuprizone and lysolecithin. Due to the complexity of MS, no single model can fully capture all its aspects (Robinson, 2014; Melamed, 2022).
See: Cuprizone Mouse Models of Multiple Sclerosis (MS)
EAE Models
The target for EAE models is the proteins expressed by oligodendrocytes, which are responsible for producing myelin in the CNS. The outcome of this model includes primary demyelination of axonal tracts, impaired axonal conduction, and progressive paralysis in the hindlimbs.
EAE can be induced in two primary ways (Constantinescu, 2011; Khan, 2014; Robinson, 2014; Terry, 2016; Hasselmann, 2017):
- Active immunization with myelin-derived proteins or peptides. Active EAE immunization can be done with several encephalitogenic epitopes, including:
- Encephalitogenic epitopes of Proteolipid Protein (PLP139-151, PLP178-191, PLP56-70)
- Myelin Basic Protein (MBP84-104, MBP12-26)
- Myelin Oligodendrocyte Glycoprotein (MOG92-106, MOG35-55, MOG1-20, MOG1-125)
- These proteins/peptides can be emulsified with complete Freund’s adjuvant (CFA) to enhance the immune response.
- Passive transfer of activated CD4+ T cells.
- Encephalitogenic CD4+ T cells targeting myelin antigens are harvested from immunized donor mice, activated in a culture, and then injected into recipient mice. This method is beneficial for developing therapies targeting specific T cell populations (e.g. Th1, Th17).
Different pathological forms of EAE arise based on the species or strain of the animal, the target protein, and the route of immunization used. For mouse models, commonly used strains include SJL (Swiss Jim Lambert) (H-2s), C57BL/6 (H-2b), PL/J, and B10.PL (H-2u). For rat models, Lewis rats and Dark Agouti strains are often used. Primates, such as marmosets, are also used as models due to their close phylogenetic relationship to humans (Linker, 2009; Khan, 2014; Robinson, 2014; Terry, 2016; Melamed, 2022).
Refer to EAE Mouse Models of Multiple Sclerosis (MS)
The typical clinical course of a mouse with EAE follows this pattern (Robinson, 2014; Hasselmann, 2017):
- A prodromal period lasting 10-15 days.
- Ascending paralysis that begins in the tail and hindlimbs.
- Progression of paralysis extending to the forelimbs.
- 15-20% weight loss in the acute phase.
- The chronic phase generally starts approximately 30 days post-induction.
- Different animal models exhibit unique characteristics of the disease. For instance:
- SJL mice (PLP139-151, MOG92-106) display a relapsing-remitting (RR) course.
- C57BL/6 mice (MOG35-55) tend to show a chronic progressive course.
- Both PL/J and B10.PL mice exhibit an acute and remitting course of disease.
- To monitor the onset, progression, and recovery from neurological damage, researchers use the EAE score, a clinical scale ranging from 0 to 5, for EAE-induced animals.
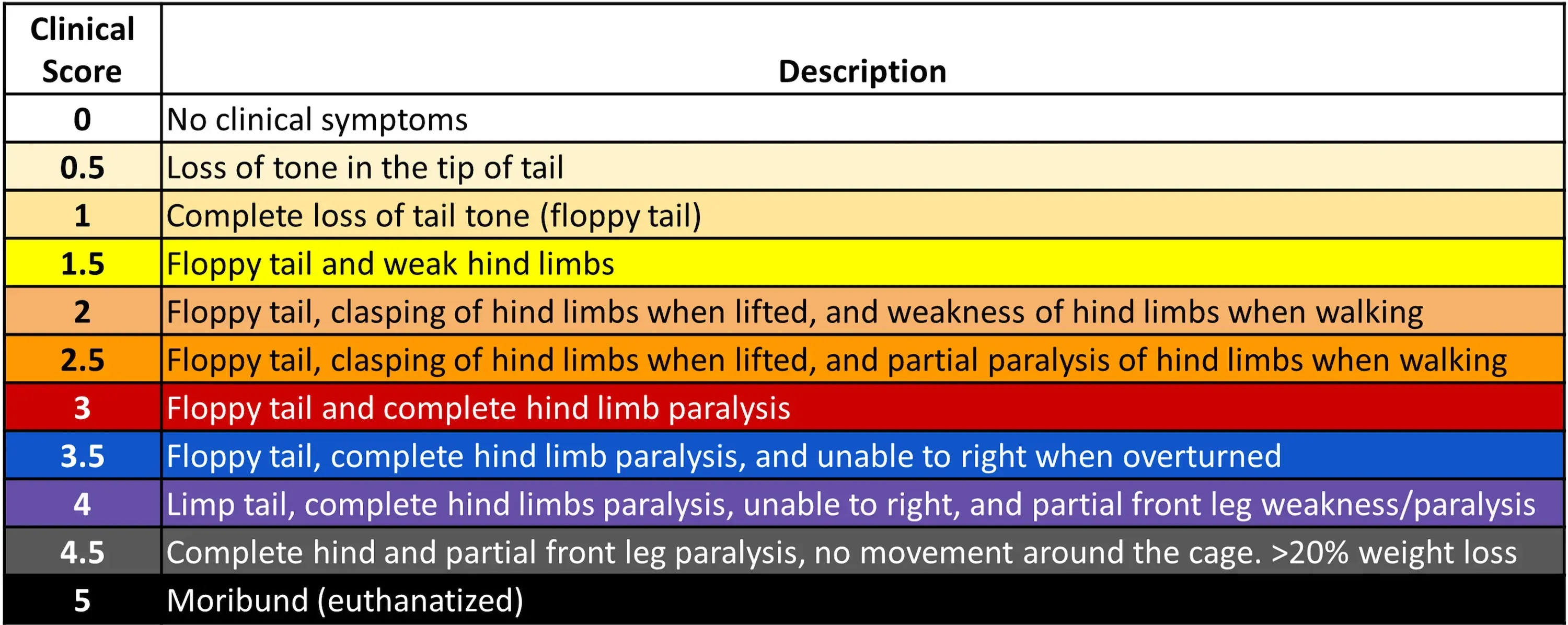
EAE Scoring System
What are the pathophysiological mechanisms involved in EAE?
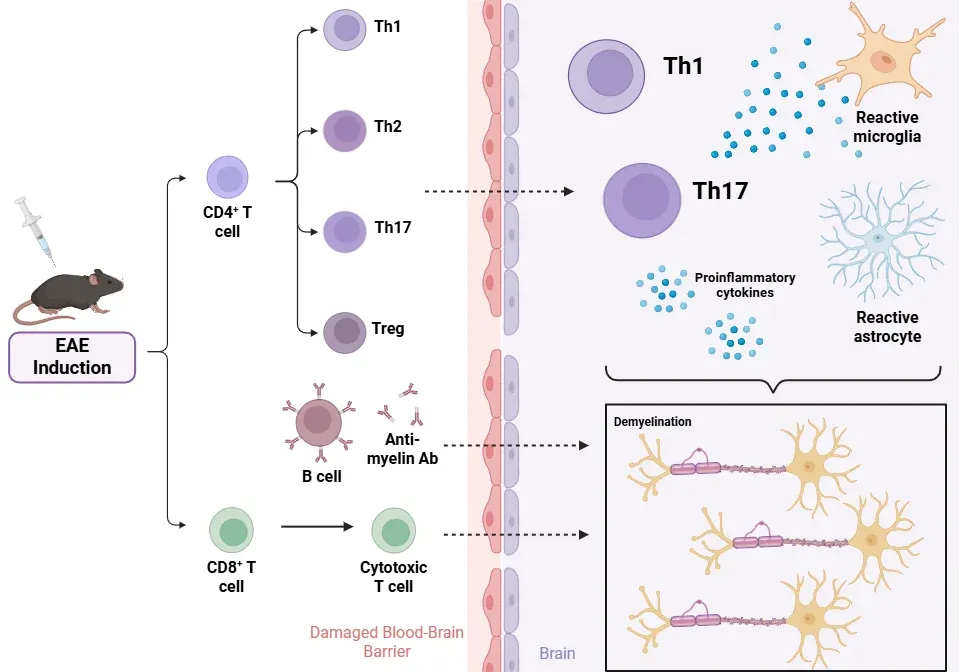
Immunohistochemistry and immunofluorescence techniques provide valuable insights into the pathological features and progression of diseases. The pathophysiology of EAE involves a complex interaction among various immune and glial cells.
CD4+ T lymphocytes (Linker, 2009; Shin, 2012; Duffy, 2014; Robinson, 2014)
- Initially, it was thought that Th1 cells were solely responsible for inducing inflammation.
- Both Th1 and Th17 cells are present in CNS lesions in EAE models. Th17 cells, driven by IL-23, alongside Th1 cells, are important in the development of EAE, though the cytokines they produce are not essential for disease onset (Th1: IFNγ and TNFα; Th17: IL-17-A, IL-17-F, IL-22).
- Th1 and Th17 cells contribute to axonal damage through mechanisms of demyelination and inflammation, which can vary based on the animal strain and the method of immunization.
- While Th1 cells are indeed pathogenic, Th2 cells play a role in immunoregulation.
- Regulatory T cells (Tregs), which secrete TGF-β and IL-10, maintain self-tolerance. Their dysfunction is linked to the progression of MS, while their activity may support spontaneous recovery in specific EAE models.
CD8+ T lymphocytes (Linker, 2009; Duffy, 2014; Robinson, 2014)
- Although EAE primarily serves as a CD4+ T cell model, CD8+ T cells and B cells are also involved in this pathology.
- CD8+ T cells can be found in MS lesions alongside microglia and macrophages, exhibiting both pathogenic and regulatory functions.
- CD8+ cytotoxic lymphocytes may directly damage myelin sheaths and axons. While they might induce EAE through CNS infiltration, their precise role remains somewhat unclear.
B cells (Robinson, 2014; Dedoni, 2023)
- B cells contribute to lesion development by acting as antigen-presenting cells and producing antibodies against myelin.
- They are vital for the onset of EAE when immunized with the MOG1-125
- B cells also play a role in T cell activation, along with dendritic cells, and can form ectopic germinal centers in the CNS of RR EAE mice.
- Differentiated B cells may release cytokines that facilitate T-cell differentiation.
- Immunoglobulin production has been detected in cerebrospinal fluid (CSF).
Pathological outcomes
- BBB disruption: IL-17, produced by Th17 cells, acts on endothelial cells, leading to the breakdown of tight junctions that maintain the barrier's integrity. This permeabilization enables the infiltration of T cells, B cells, and macrophages (Shin, 2012; Duffy, 2014; Dhaiban, 2021).
- Inflammation and demyelination: The infiltration of immune cells into the CNS causes local inflammation and edema, leading to the destruction of the myelin sheath surrounding axons (Robinson, 2014; Toader, 2018).
- Axonal injury: Widespread axonal damage occurs in conjunction with demyelinating lesions, which contribute significantly to progressive neurological deficits (Robinson, 2014; Hasselmann, 2017).
- Gliosis: In response to inflammation and tissue damage, astrocytes become reactive and lead to the formation of glial scars (Toader, 2018).
- Mitochondrial dysfunction: Mitochondrial dysfunction contributes to nitric oxide free radical production, affecting EAE pathogenesis. Correlation exists between mitochondrial dysfunction and cortical lesions in some MS patients (Dutta, 2006).
Pathophysiology of EAE
What role do positive controls play in preclinical studies using EAE models?
Positive controls are necessary in EAE research to validate the experimental model's responsiveness to known effective treatments and to provide a reliable target against which novel therapeutic agents can be compared (Robinson, 2014; Melamed, 2022). Below are key positive controls used in EAE studies.
Clinical course of chronic and relapsing-remitting EAE and the effect of positive controls
Dexamethasone (Donia, 2010; Nam, 2021)
- A glucocorticoid used to treat acute relapses in multiple sclerosis (MS) patients and serves as a standard positive control, presenting immunosuppressive and anti-inflammatory effects in EAE.
- It binds to cytosolic glucocorticoid receptors, which translocate to the nucleus to regulate gene expression. This action suppresses pro-inflammatory pathways, such as NF-κB, while activating anti-inflammatory genes.
- It preserves myelin and axons, inhibiting the infiltration of peripheral immune cells (CD45+) and delaying the activation of microglia and astrocytes in the early stages of the disease.
- Dexamethasone significantly postpones the clinical onset of EAE and reduces the peak severity of the disease.
- The protective effects of dexamethasone are transient, and glucocorticoid resistance may develop.
Fingolimod (Webb, 2004; Choi, 2011; Constantinescu, 2011; Robinson, 2014; Melamed, 2022)
- Fingolimod was the first FDA-approved oral disease-modifying treatment (DMT) for MS and is highly effective in EAE.
- Fingolimod serves as a positive control for testing therapies that target leukocyte trafficking, cell migration from lymphoid organs, or sphingosine-1-phosphate receptor 1 (S1P₁) signaling pathways.
- The primary mechanism involves the phosphorylation of FTY720 to its active form, FTY720-P. This molecule causes the internalization of SIP₁ on lymphocytes, trapping them within secondary lymphoid organs and preventing their migration into the CNS. Fingolimod also affects S1P₁ on astrocytes within the CNS.
- Fingolimod almost completely prevents the development of EAE in Lewis rats.
Interferon-beta (IFN-β) (Constantinescu, 2011; Robinson, 2014)
- A first-line injectable DMT for MS, IFN-β serves as a positive control for immunomodulatory therapies in EAE and is intended to modulate cytokine balance, particularly in Th1 and Th17 cells.
- IFN-β ameliorates symptoms, progression, and development of EAE by limiting IFN-γ (from Th1 cells) and IL-17 (from Th17 cells) pro-inflammatory responses in vivo.
Natalizumab (Constantinescu, 2011; Robinson, 2014)
- Natalizumab is a monoclonal antibody against the α4β1 integrin (VLA-4). It blocks lymphocyte adhesion to blood vessels, preventing their entry into the CNS.
- It serves as a positive control for therapies targeting leukocyte trafficking and CNS infiltration.
Glatiramer Acetate (Schrempf, 2007; Constantinescu, 2011; Moore, 2014; Robinson, 2014)
- Glatiramer acetate is a synthetic analogue of MBP composed of various polypeptides that modulate the immune response of T-cells and promote the survival of oligodendrocyte precursor cells.
- It has been shown to reduce relapses in RR-MS by approximately 30%.
Dimethyl Fumarate (DMF) (Dhaiban, 2021; Melamed, 2022)
- DMF is an FDA-approved oral DMT with demonstrated benefits in EAE models.
- It modulates Th1 and Th2 responses, inhibits pro-inflammatory cytokines, and enhances NK cell function.
The EAE model offers valuable insights into the mechanisms of demyelination, particularly in the context of spinal cord involvement. While it primarily focuses on spinal cord demyelination, which differs from the broader cerebral and cerebellar lesions observed in MS, it remains a useful tool for understanding disease progression. Furthermore, while the emphasis on CD4+ T cells is prominent in EAE, ongoing investigations are increasingly recognizing the importance of CD8+ cytotoxic T cells in MS. This evolving perspective highlights the potential for improving therapeutic strategies obtained from EAE research to better translate to clinical applications in MS. The continued exploration in this area of neuroscience holds promise for advancing our understanding and treatment options.
Our team would be happy to answer any questions about EAE models or provide specific information about the models that we use for therapeutic efficacy studies.
Discover more about our MS Models
Related Content
Up-to-date information on Multiple Sclerosis and best practices related to the evaluation of therapeutic agents in MS animal models.
Demyelination & Remyelination in the Cuprizone Model
An overview of the methods available to measure myelin and oligodendrocytes in the cuprizone demyelination mouse model of multiple sclerosis (MS).
Experimental Autoimmune Encephalomyelitis (EAE) & Axonal Injury
This resource describes the methods available for measuring axonal damage & axon degeneration, including tissue markers and plasma & CSF neurofilament light chain (NfL; NF-L) levels, in the EAE model of multiple sclerosis (MS).
Mitochondrial Dysfunction in Microglia & Astrocytes
The role of mitochondrial dysfunction in microglia and astrocytes in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.