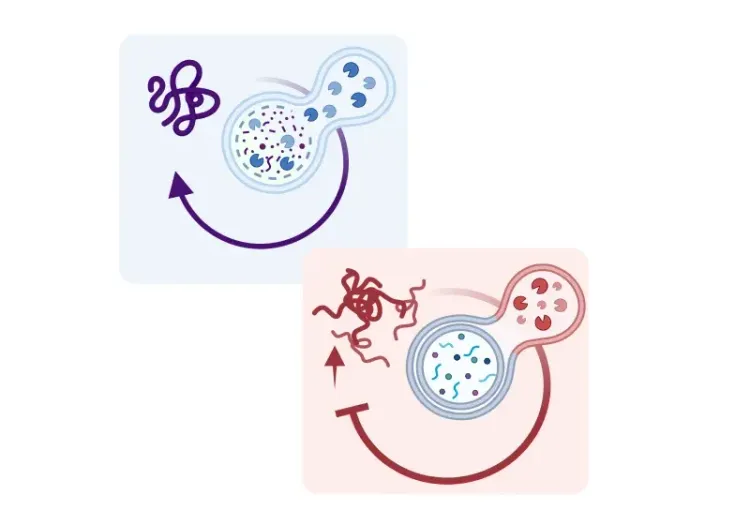
What is Transcription Factor EB (TFEB)?
Transcription factors (TFs) are a group of proteins that regulate gene expression by binding to specific DNA sequences in order to inhibit or promote the process of DNA transcription into RNA, effectively turning genes “on” and “off” (Wang, 2015; Oksuz, 2023). Discovered in 2009, Transcription Factor EB (TFEB) belongs to the microphthalmia-associated transcription factor/transcription factor E (MiTF/TFE) family, which plays diverse roles in nutrient sensing, fat and sugar metabolism, autophagy, organelle formation and function, and adaptive response to a range of cellular stressors, including oxidative stress, microbial infection, and mitochondrial damage (La Spina, 2021; Gebrie, 2023).
Originally described as an oncogene, TFEB is now recognized as a master regulator of the autophagy-lysosome pathway. This pathway is crucial for the delivery of cellular cargo, such as protein aggregates, dysfunctional organelles, lipids, and other components, to the lysosome for degradation and synthesis of new molecules. TFEB promotes autophagy by upregulating genes responsible for autophagosome formation, lysosome biogenesis, lysosome function, lipid metabolism, and mitochondrial quality control (Sardiello, 2009; Napolitano, 2016; Cortes, 2020; Chen, 2024). TFEB signaling is activated under various stress conditions, such as starvation, oxidative stress, lysosomal damage and dysfunction, protein aggregation, and mitochondrial damage (Wang, 2020; Franco-Juarez, 2022).
In the central nervous system (CNS), TFEB signaling confers additional benefits. It reduces neuroinflammation and promotes neuroplasticity by enhancing clearance of intracellular debris, rescuing synaptic function, and regulating neuronal metabolic health (Wang, 2020; Gu, 2022; Matthews, 2023). A recent study by Matthews et al. (Matthews, 2023) demonstrated these benefits by overexpressing TFEB in the skeletal muscles of cTFEB;HSACre transgenic mice. This muscle-specific TFEB overexpression reduced brain inflammation, improved biomarkers of aging in the hippocampus, and enhanced expression of genes regulating synaptic transmission, neuroplasticity, and inflammation response.
For visualization and quantification of TFEB activity, in vivo studies in mice and live cells often combine cellular imaging with biochemical assays, using molecular reporters, such as luciferase and tdTomato. Since TFEB’s translocation from the cytoplasm to the nucleus is regulated by phosphorylation, Western blot is a key technique for distinguishing between its phosphorylated (active) and non-phosphorylated (inactive) forms (Brunialti, 2024).
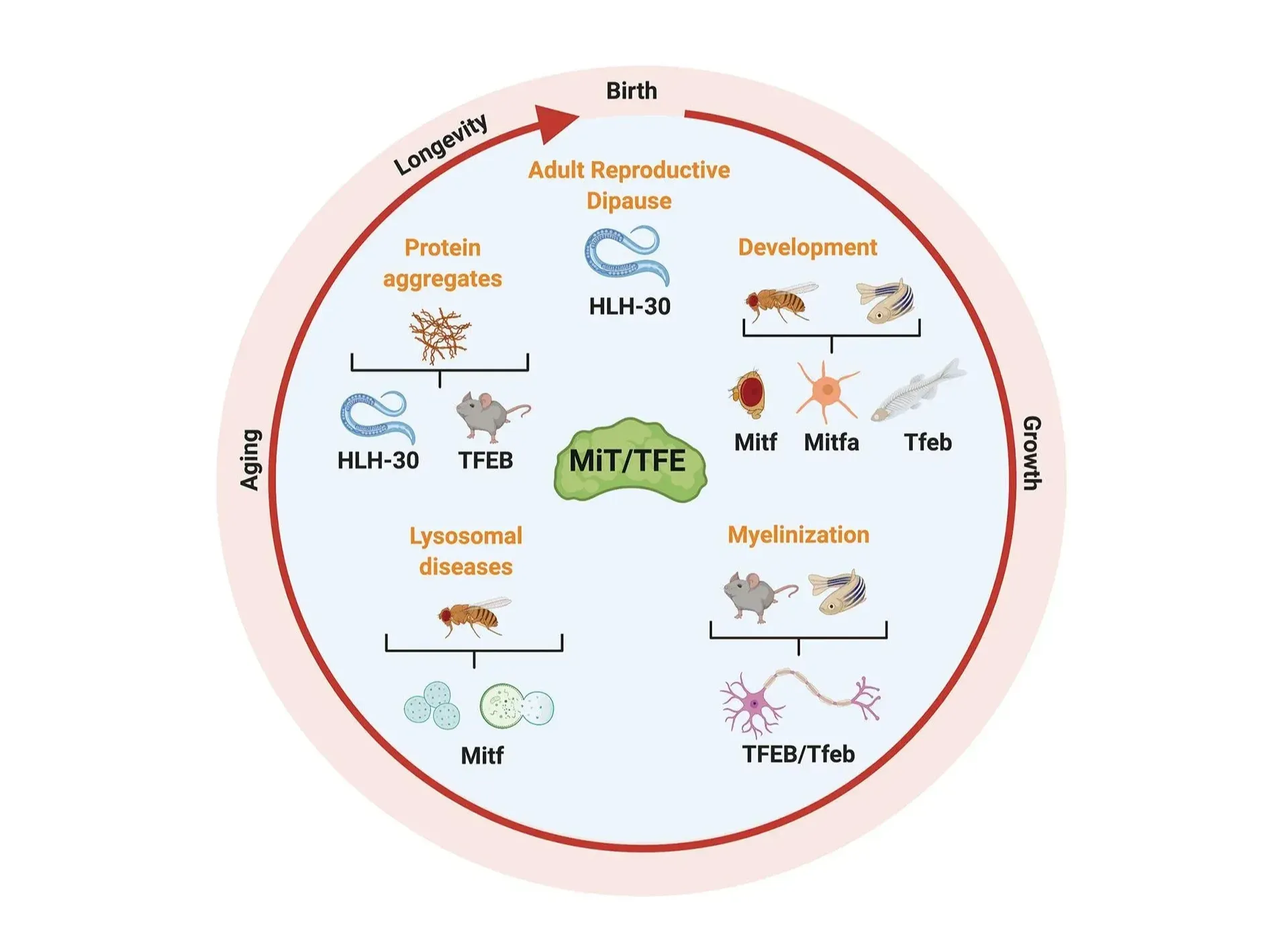
The members of the MiT/TFE family play critical roles in various biological processes, including development, longevity, and stress response. Figure and caption are reproduced from La Spina et al. (La Spina, 2021) under the Creative Commons Attribution License.
What is the relationship between TFEB and autophagy?
Impaired autophagy is closely associated with the pathology of several neurodegenerative diseases, including Alzheimer’s (AD), Parkinson’s disease (PD), Amyotrophic Lateral Sclerosis (ALS), and Huntington’s disease (HD) (Nah, 2015; Deng, 2017; Menzies, 2017).
For an in-depth review on the role of impaired autophagy in neurodegenerative diseases, see: Autophagy & Neurodegenerative Diseases
As a master regulator of autophagy, TFEB upregulates genes responsible for lysosomal biogenesis and function (Palmieri, 2011; Settembre, 2011; Song, 2021). These genes are part of the CLEAR—Coordinated Lysosomal Expression and Regulation—network, and share a common DNA sequence, called the CLEAR motif, in their promoters. TFEB directly binds to this 10 base E-box sequence to induce autophagosome biogenesis and autophagosome–lysosome fusion (Palmieri, 2011; Settembre, 2011; Chen, 2024).
Due to its role as a master regulator of autophagy, TFEB dysfunction acts as a driver for neurodegeneration (Martini-Stoica, 2017; Cortes, 2019; Song, 2021). Studies in mouse and cellular models have shown that overexpression of TFEB promotes the clearance of protein aggregates (Decressac, 2013; Polito, 2014; Chauhan, 2015). However, when the activity of the CLEAR network is reduced or impaired due to cytosolic retention of TFEB, it contributes to the pathophysiology of neurodegenerative diseases (Reddy, 2016). Tiribuzi et al. (Tiribuzi, 2014) reported significant alterations in TFEB function and localization in Alzheimer’s disease. Their analysis of AD patient lymphocytes and monocytes revealed reduced expression of several lysosomal enzymes, indicating that TFEB dysfunction may be associated with lysosomal deficits observed in the disease process.
It is also worth noting that TFEB’s relationship with autophagy is complex, and its activity is regulated through multiple links, including protein-protein interactions. mTORC1, an inhibitor of autophagy, directly phosphorylates TFEB, preventing its activation and nuclear translocation. When mTORC1 activity is reduced or inhibited, it can no longer phosphorylate proteins like TFEB. This scenario leads to TFEB’s translocation to the nucleus, where it binds to CLEAR promoters, triggering autophagy (Martina, 2012; Zhang, 2020; Franco-Juarez, 2022).
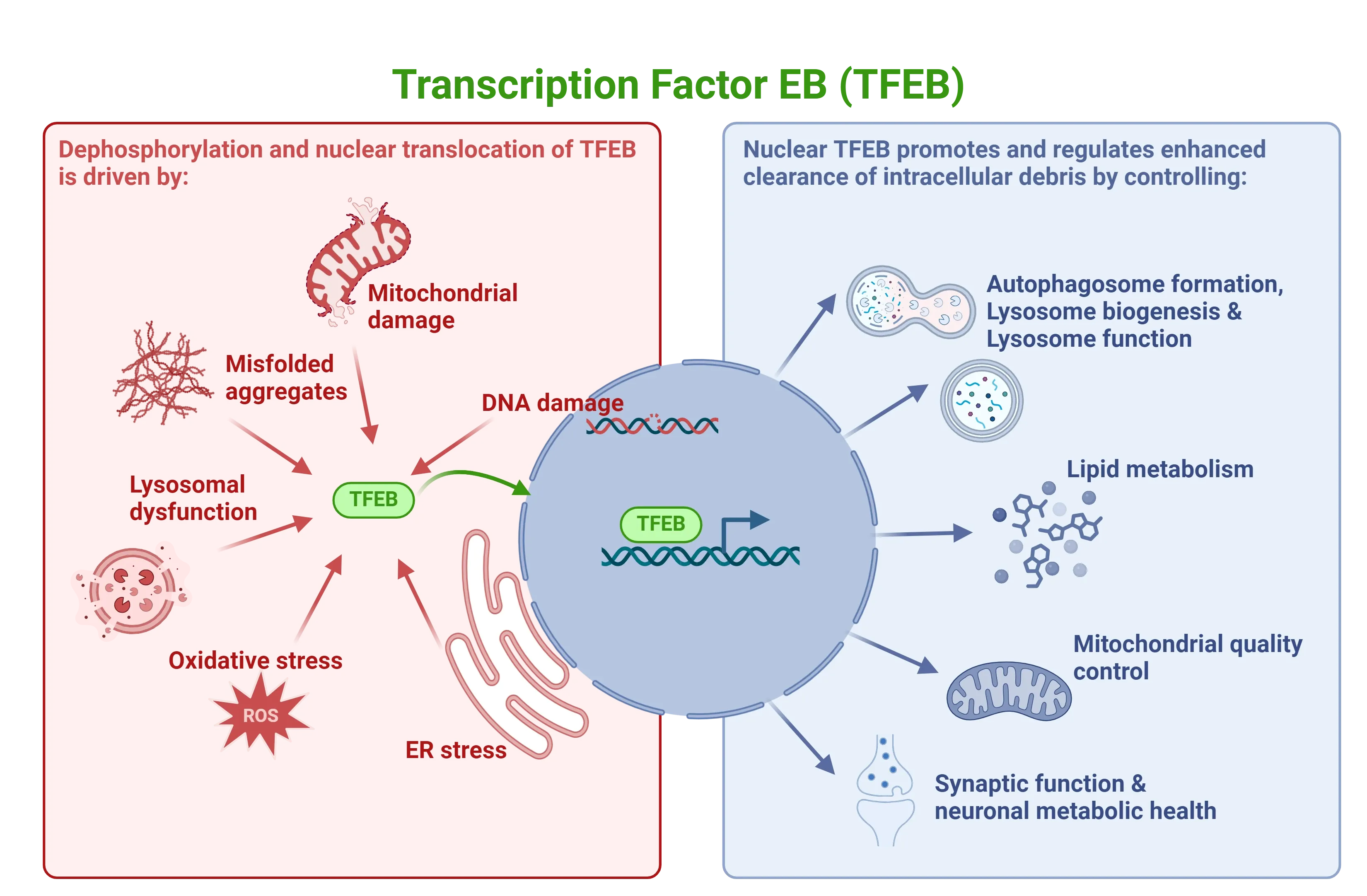
TFEB signaling is activated under various stress conditions, such as oxidative stress, lysosomal damage and dysfunction, protein aggregation, and mitochondrial damage. Upon nuclear translocation, TFEB promotes autophagy by upregulating genes responsible for autophagosome formation, lysosome biogenesis, lysosome function, lipid metabolism, mitochondrial quality control, and neuronal metabolic health.
Have TFEB-mediated approaches demonstrated therapeutic potential in neurodegenerative diseases?
TFEB’s activation is a promising therapeutic strategy for neurodegenerative diseases, with studies demonstrating its ability to reliably enhance autophagic flux across multiple neurodegenerative disease models.
Amyotrophic Lateral Sclerosis (ALS)
Young et al. (Young, 2023) have demonstrated the ability of a small molecule selective PIKfyve inhibitor, AIT-101 (INN: apilimod, aka LAM-002A), which has been shown to activate TFEB, to decrease the loss of body weight, reduce motor deficits (including hindlimb clasping, hindlimb paralysis, and grill agility), along with accompanying decreases in plasma and CSF neurofilament light (NfL) levels, TDP-43 aggregates (by IHC), and neuroinflammation (by GFAP IHC) in Biospective's Low Dox version of the rNLS8 model of ALS.
Alzheimer’s Disease (AD)
In mice with five familial AD mutations (5xFAD), recent work by Chun et al. (Chun, 2022) demonstrated that oral administration of trametinib reduces amyloid-beta deposits, improving cognitive function, and repairing impaired neural structures. Trametinib achieves this disease modification by inhibiting TFEB phosphorylation, allowing it to translocate to the nucleus, where it regulates the expression of CLEAR genes and activates autophagy. Polito et al. (Polito, 2014) also found that activating TFEB through an adeno-associated virus (AAV) delivery system reduces neurofibrillary tangles—tau protein aggregates in AD—in the brains of rTg4510 mice (Polito, 2014).
Parkinson’s Disease (PD)
Rapamycin, an inhibitor of mTOR, is shown to promote autophagy in PD mouse models. Bai et al. (Bai, 2015) demonstrated that feeding a rapamycin diet to Atg5 alpha-synuclein transgenic mice for 24 weeks improved motor function and reduced the loss of synaptophysin protein, which is commonly diminished in PD, through TFEB’s activation of autophagy. Similarly, Decressac et al. (Decressac, 2013) found that TFEB-mediated autophagy has a neuroprotective effect on A9 and A10 dopaminergic neurons in an in vivo model of alpha-synuclein toxicity.
See: Autophagy, Parkinson's Disease, and Dopaminergic Neurons
Huntington’s Disease (HD)
In a transgenic mouse model of HD, Tsunemi et al. (Tsunemi, 2012) found that overexpression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) clears mutant huntingtin (mHTT) aggregates and improves neural function in HD mice. PGC-1α is known to regulate cellular energy metabolism and activate TFEB, which, in turn, enhances autophagic flux. Similarly, Vodicka et al. (Vodicka, 2016) demonstrated that directly expressing TFEB in the striatum of HD Q175/Q7 mice stimulates autophagy, lowering mHTT levels. Together, these findings suggest that TFEB activation can alleviate toxic protein aggregates in HD models.
Our team would be happy to answer any questions about autophagy and Transcription Factor EB (TFEB) in neurodegenerative diseases or provide specific information about the AD, PD, and ALS models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on autophagy and Transcription Factor EB (TFEB) in neurodegenerative diseases and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Impaired Microglia Autophagy in Neurodegenerative Diseases
How impaired microglia autophagy contributes to the progression of neurodegenerative diseases.
Lysosome Dysfunction in Microglia & Astrocytes
An overview of lysosomal dysfunction in microglia & astrocytes, and its role in neurodegenerative diseases.
Autophagy & Neurodegenerative Diseases
An overview of how cellular autophagy plays a role in brain health and neurodegeneration.
Autophagy, Parkinson's Disease, and Dopaminergic Neurons
An overview of how impaired autophagy can lead to pathologic changes and neurodegeneration in dopaminergic neurons in Parkinson’s disease.