What is the PS19 mouse model?
Tauopathies are a group of neurodegenerative disorders characterized by the abnormal aggregation of tau, a microtubule associated protein that helps maintain the cellular architecture of neurons. In these disorders, tau becomes hyperphosphorylated, which causes it to detach from microtubules and accumulate inside neurons; the resulting aggregation destabilizes the neuronal cytoskeleton, leading to neurotoxicity and eventual cell death (Wang, 2016). To date, over 26 distinct tauopathies have been identified, including Alzheimer’s disease, frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), and progressive supranuclear palsy (Sexton, 2022).
Research on tau and its involvement in neurodegeneration is progressing quickly. Advances in technology and the generation of animal models that accurately replicate tauopathy phenotypes have enabled the development of drugs targeting tau accumulation and spread, as well as research into their effects on associated motor, cognitive, and behavioral outcomes (Soeda, 2020).
The PS19 model is one of the most widely used mouse models for studying neurodegenerative tauopathies. PS19 mice express human tau with the P301S mutation – a missense mutation in the tau gene – driven by the mouse prion protein promoter (PrP). This strong, constitutive promoter drives tau expression to levels approximately five times higher than endogenously produced (Yoshiyama, 2007). Additionally, P301S alters tau’s protein conformation, making it more accessible to kinases that phosphorylate it at multiple sites. The resulting hyperphosphorylation promotes tau’s detachment from microtubules and enhances both the aggregation capability and seeding potency of tau molecules (Strang, 2019).
Numerous animal models exist for studying tauopathies; however, the PS19 model is one of the preferred models for drug development targeting tau aggregation and spread (Götz, 2019). This model offers a broad range of clinically relevant phenotypes that recapitulate those observed in human tauopathies, including:
- Progressive tau aggregation
- Neuroinflammation
- Motor deficits
- Cognitive impairment
The P301S mutation also drives pathology in multiple brain regions, including:
- Cerebral cortex
- Hippocampus
- Brainstem
- Spinal cord
This broad spectrum of pathology better mimics the spatial aspect of disease as seen in conditions like Alzheimer's disease and FTDP-17, enhancing the model’s utility for studying both cognitive and motor symptoms (Alves, 2025).
The most important characteristics of the PS19 model that make it suitable for testing multi-target disease-modifying therapies include:
- Robust and progressive tau seeding and spreading bioactivity that corresponds with cognitive and motor impairments
- A strong neuroinflammation profile with both microgliosis and astrogliosis
- Widespread neuronal cell death and brain atrophy across multiple brain regions
- Age-associated cognitive impairment
- Pronounced motor decline and muscle weakness at later stages of pathology
- Reduced survival
What neuropathologic changes can be observed in this model?
- Around 3 months of age, PS19 mice begin to develop early signs of tau pathology, including synaptic dysfunction and microgliosis.
- By 5 to 6 months, intracellular aggregates of hyperphosphorylated tau – known as neurofibrillary tangles (NFTs) – begin to appear in multiple brain regions, including the cortex, hippocampus, amygdala, brainstem, and spinal cord.
- Around 6 months of age, PS19 mice begin to exhibit a progressive increase in the release of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α; this cytokine elevation promotes astrogliosis and microglial activation, which exacerbate neurodegenerative process and can be detected using various immunoassays (Jati, 2025).
- By 8 months, these mice develop overt neuronal loss and hippocampal atrophy, which gradually extends to other brain regions such as the neocortex and entorhinal cortex by 9 months, as revealed by volumetric magnetic resonance imaging (MRI) (Yoshiyama, 2007). The pathology is also marked at this stage by widespread neuroinflammation, mitochondrial dysfunction, and increased production of reactive oxygen species.
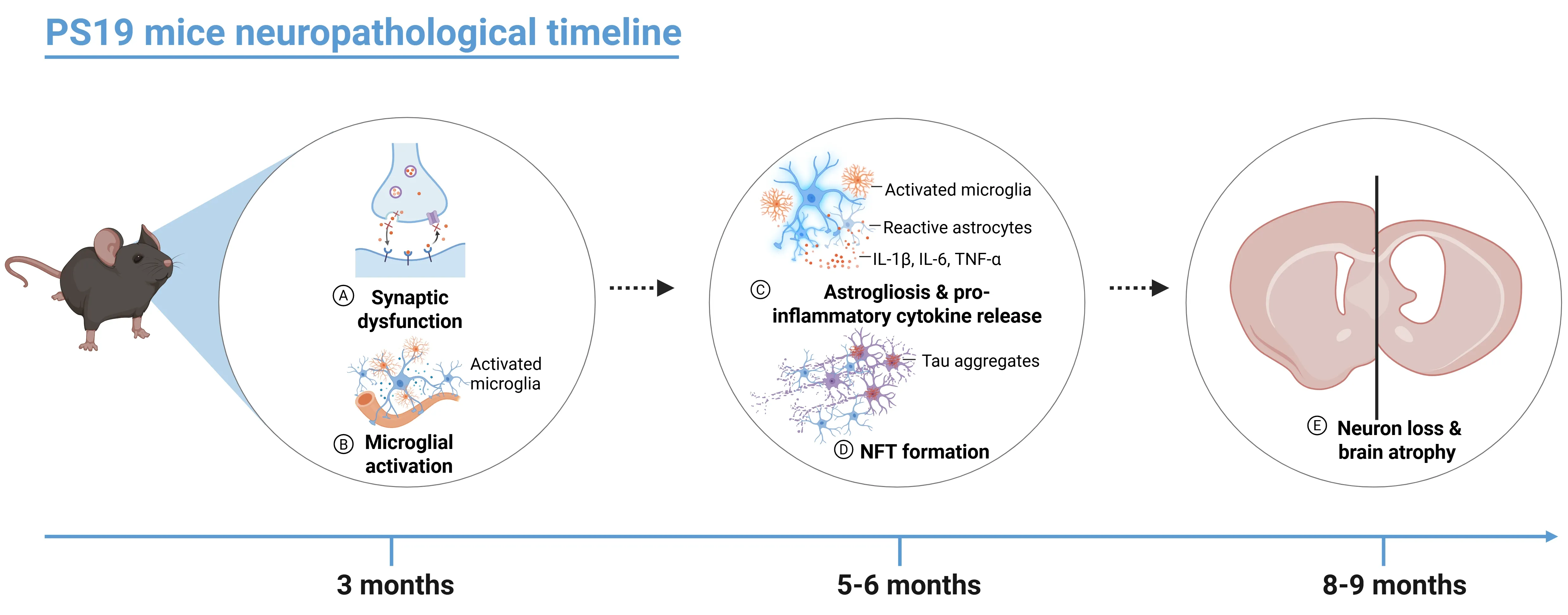
Timeline of onset of neuropathological changes in PS19 (P310S) mice.
Tau pathology in PS19 mice can also be experimentally induced through stereotaxic injection of tau preformed fibrils (PFFs). When delivered to the hippocampus and overlying cortex, tau PFFs rapidly seed NFT-like aggregates at the injection site. These aggregates then spread to connected brain regions via neuronal networks; the induced pathology closely resembles tau pathology in Alzheimer’s disease (Iba, 2013), exhibiting key features such as:
- Increased tau phosphorylation and acetylation
- β-sheet amyloid structures (Thioflavin S-positive)
- Resistance to proteinase K digestion
This neuropathologic profile makes PS19 a suitable model for drug development targeting tauopathy in diseases like Alzheimer’s disease and frontotemporal dementia. It recapitulates many pathological and clinical features of these tauopathies, making it a valuable platform for drug discovery aimed at modifying tau-related mechanisms.
At Biospective, our stereotaxic delivery of sonicated, recombinant human tau PFFs or brain extracts into the hippocampus and overlying cortex of PS19 mice helps accelerate and synchronize the onset of tau pathology in this model, compared to the spontaneous, age-dependent development seen in PS19 mice without injection.
Common neuropathologic outcomes can be detected in this model using:
- Volumetric magnetic resonance imaging (MRI) to measure brain atrophy and ventricular enlargement
- Immunohistology staining, imaging, and analysis to detect neuron loss, astrogliosis, microgliosis, and spread of tau pathology.
- ELISA and related immunoassay-based technologies to detect changes in production of inflammatory cytokines such as IL-1β, IL-6, and TNF-α.
Which cognitive and motor phenotypes do PS19 mice exhibit?
PS19 mice develop progressive cognitive and motor deficits that recapitulate phenotypes observed in human tauopathies. At around 6 months of age, cognitive deficits particularly in memory and learning typically begin to emerge (Ahmad, 2021). These impairments can be observed in tasks including:
- Novel object recognition test
- Y-maze
- Elevated plus maze
- Morris water maze
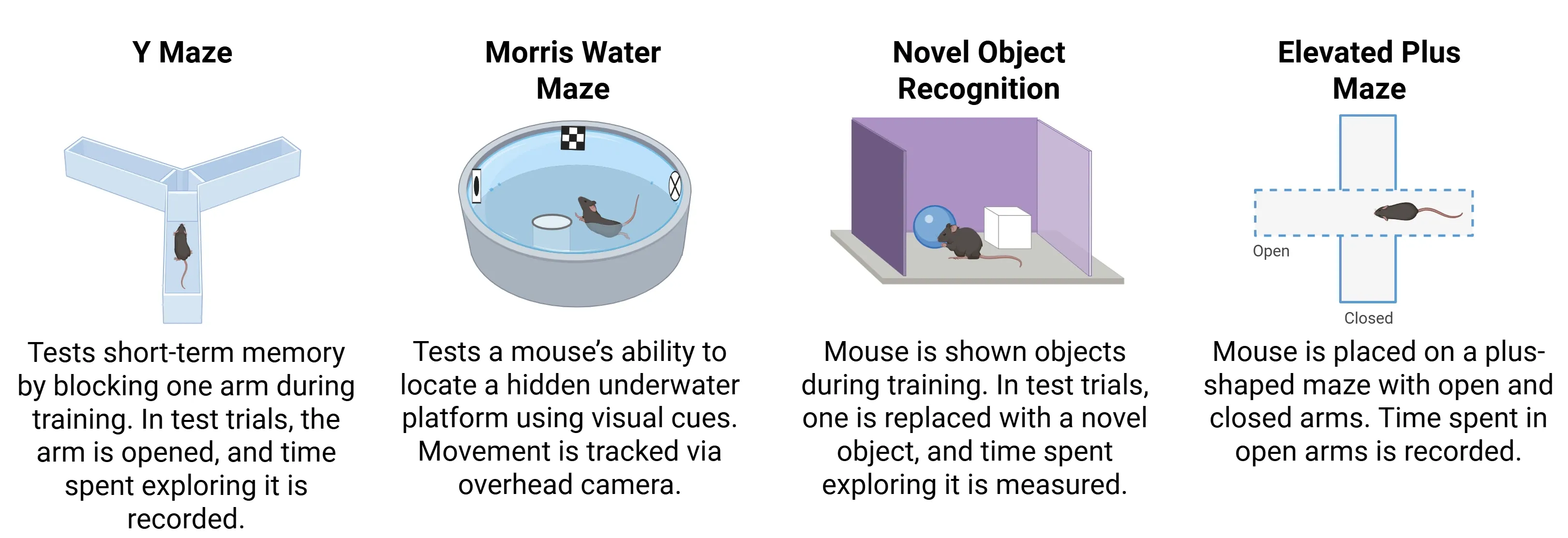
Common tests used to assess cognitive changes in PS19 mice.
In addition to cognitive deficits, PS19 mice develop distinct locomotor and physiological abnormalities as tau pathology advances (Dumont, 2011; Patel, 2022). By 9 months of age, these mice show a marked increase in locomotor activity associated with hyperactivity and behavioral disinhibition, commonly assessed using a variety of motor tests, including:
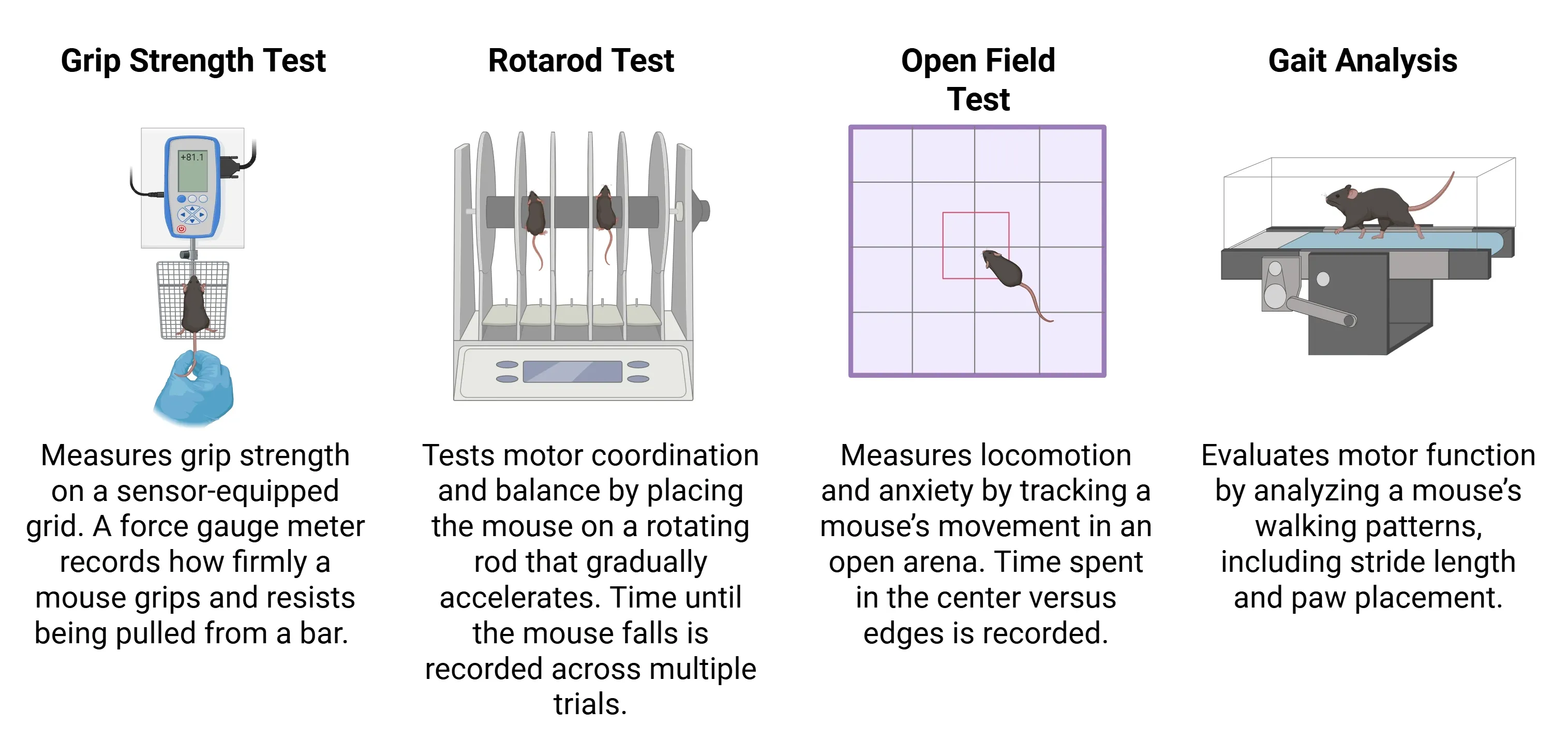
Common tests used to assess motor phenotypes in PS19 mice.
At the same time, PS19 mice begin to exhibit motor weakness alongside clear signs of systemic decline, including weight loss, reduced body temperature, and increased frailty (Patel, 2022). These features reflect the broader physiological impact of tau pathology and closely parallel aspects of physical deterioration observed in human tauopathies.
These cognitive and motor phenotypes can be further accelerated and synchronized by stereotaxic injection of tau preformed fibrils (PFFs). When PFFs are introduced into the hippocampus and overlying cortex, mature, NFT-like tau aggregates form as early as one-month post-injection (Iba, 2015). Tau PFF-injected PS19 mice develop earlier and more robust cognitive impairments, including memory and learning deficits, compared to non-injected mice, in which such deficits typically appear around 6 months. Tau PFF stereotaxic delivery also leads to earlier neuronal loss in regions such as the locus coeruleus and correlates with premature onset of motor deficits.
Together, these cognitive, behavioral, and motor phenotypes make the PS19 model a powerful platform for evaluating the effects of tau-targeting therapies.
Our team would be happy to answer any questions about the PS19 Mouse Model for Tau Targeted Drug Development or provide specific information about the models we use for therapeutic efficacy studies.
Discover more about our Neurodegenerative Diseases Models
Related Content
Up-to-date information on Neuroinflammation and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Neuroimaging in Frontotemporal Dementia & Clinical Trials
The utility of MRI & PET imaging biomarkers in our understanding of Frontotemporal Dementia (FTD) variants, and their use as endpoints in FTD clinical trials.
Imaging Biomarkers for Progressive Supranuclear Palsy (PSP)
An overview of the various brain imaging methods (MRI, PET, SPECT) available to assess efficacy of disease-modifying therapeutics in clinical trials of PSP.
Longitudinal Change in Tau PET in MCI & Alzheimer’s Disease
An overview of the natural history of change in Tau PET tracer uptake & binding in Mild Cognitive Impairment (MCI) & Alzheimer’s disease (AD).
Frontotemporal Dementia (FTD) & MRI Brain Atrophy
Neuroimaging biomarkers (including MRI brain atrophy) from the FTLDNI natural history study of Frontotemporal Dementia (FTD).