What is the relationship between autophagy and alpha-synuclein pathology?
Alpha-synuclein is a presynaptic neuronal protein. Mutant versions of alpha-synuclein, implicated in Parkinson’s disease, tend to misfold and aggregate within neurons to form insoluble fibrils that disrupt cellular processes (Vidović, 2022; Negi, 2024). Chaperone-mediated autophagy (CMA), a selective form of autophagy for the degradation of cytosolic proteins, is a major pathway for the degradation of alpha-synuclein (Vogiatzi, 2008; Vidal, 2014; Frake, 2015). Hence, disruption of CMA and other autophagy pathways is closely linked with accumulation of alpha-synuclein within cells and PD pathology.
Several autophagy-regulating proteins, such as Leucine-Rich Repeat Kinase 2 (LRRK2), PTEN-Induced Kinase 1 (PINK1), and Parkin RBR E3 Ubiquitin Protein Ligase (PARKIN), interact with alpha-synuclein (Beilina, 2015; Hou, 2020; Zhang, 2022). Mutations in the gene encoding Parkin, a protein involved in the removal of damaged mitochondria and unnecessary proteins, are linked to early onset PD in primate models (Han, 2024). PINK1 interacts with alpha-synuclein in the cytoplasm through its kinase domain, and PINK1 mutations exacerbate alpha-synuclein aggregation and neuroinflammation in PINK1 knockout mice (Nguyen, 2022). Similarly, loss of LRRK2 impairs protein degradation pathways, leading to increased alpha-synuclein accumulation and programmed cell death in mouse models (Tong, 2010).
Mutant forms of alpha-synuclein can also disrupt autophagic processes. When Winslow et al. (Winslow, 2010) overexpressed wild-type alpha-synuclein in mammalian cells and M7 alpha-synuclein transgenic mice, it resulted in the inhibition of Rab1, a critical autophagy regulator. This change led to the mislocalization of Atg9, disrupting the formation of omegasomes—sites where autophagosomes form.
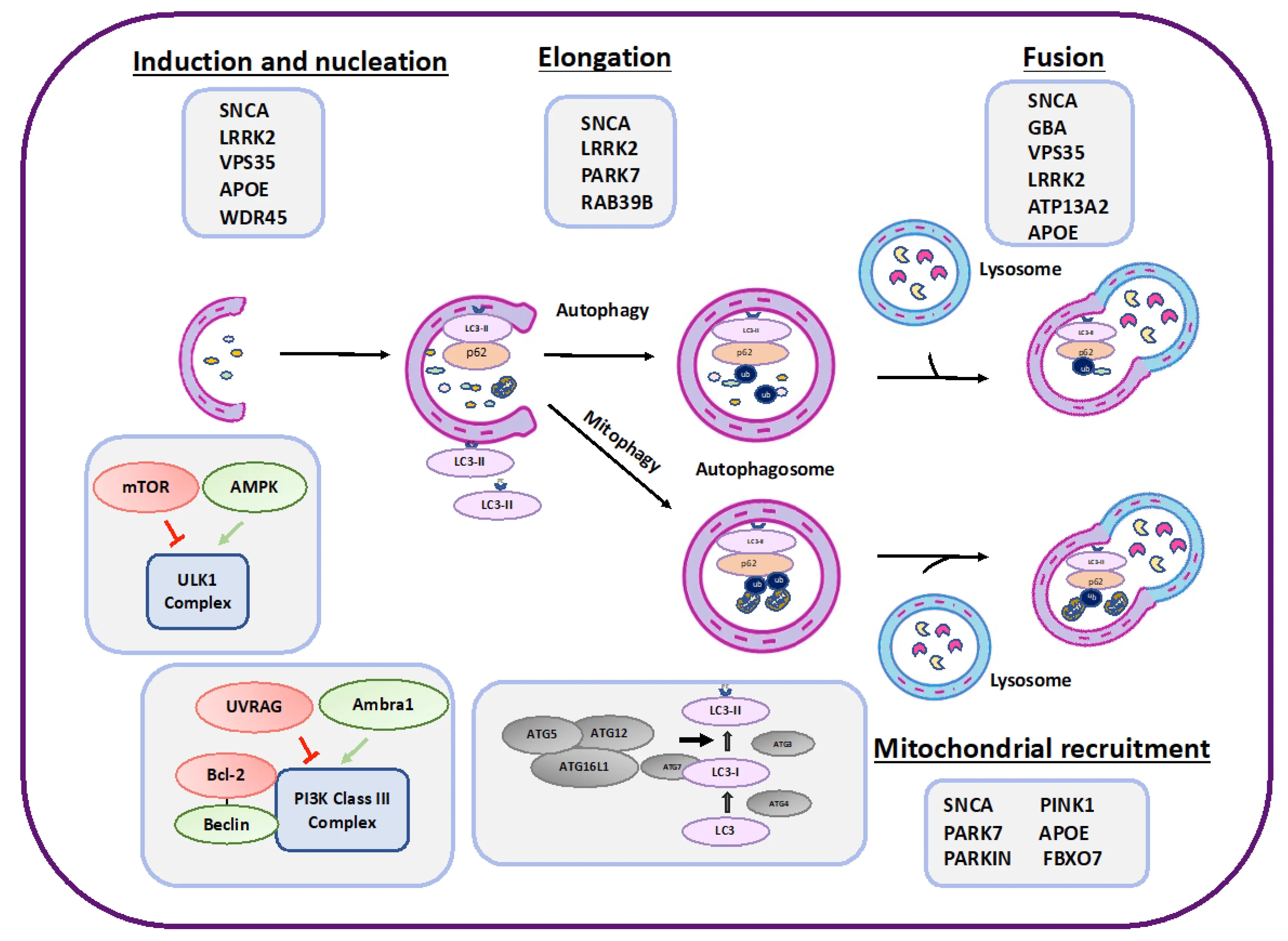
A schematic presentation of proteins involved in various stages of autophagy that may also be playing a role in PD (gray boxes). Negative regulators of autophagy are represented in red, while positive regulators are in green. Figure reproduced from Nechushtai et al. (Nechushtai, 2023) under the Creative Commons Attribution License.
What is the association between autophagy and degeneration of dopaminergic neurons?
Autophagy is essential for the health and survival of dopaminergic neurons in the substantia nigra, as inhibition of autophagy can lead to axonal injury, dendritic degeneration, and cell death (Friedman 2012; Yang, 2013). Loss of autophagy in dopaminergic neurons of Atg5 and Atg7 knockout mice has been shown to cause motor dysfunction through degeneration of dopaminergic neurons and the formation of Lewy body-like structures—hallmarks of PD (Friedman, 2012; Ren, 2018; Sato, 2018).
Dopaminergic neurons have a high energy requirements because of their vast and complex axonal and dendritic network. Given the bi-directional relationship between alpha-synuclein aggregation and autophagy, alpha-synuclein aggregates impair the autophagy-lysosome pathway, leading to insufficient clearance of dysfunctional mitochondria. Chronic energy depletion can lead to axonal degeneration and cell death, as dopaminergic neurons are unable to power essential cellular processes (Bolam, 2012; Moors, 2016; Xilouri, 2016).
Additionally, impaired macroautophagy—a type of stress-induced autophagy—leads to enhanced dopamine neurotransmission. The effects of this are complex: temporary improvement in motor function due to the enhanced dopamine signaling, but accelerated disease progression (Hernandez, 2012; Hunn, 2019).
Autophagic degeneration has been observed in dopaminergic neurons of PD patients. Anglade et al. (Anglade, 1997) conduced an ultrastructural analysis of PD brains and found features consistent with autophagic degeneration in dopaminergic neurons: vacuoles containing cytoplasmic material, fragmented nuclei with condensed chromatin, and lysosome-like vacuoles. Signs of necrosis were not detected in these neurons, further suggesting autophagic degeneration as the primary cause of cell death.
For an in-depth review of the role of autophagy in neurodegenerative diseases, see: Autophagy & Neurodegenerative Diseases
See also: Impaired Microglia Autophagy in Neurodegenerative Diseases
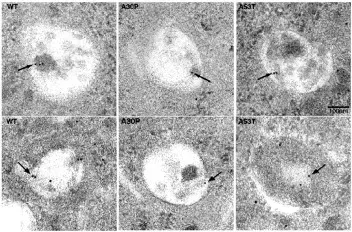
Immunogold electron microscopy in PC12 cell lines points to localization of alpha-synuclein in vesicles with autophagic features. Figure is reproduced from Webb et al. (Webb, 2003) under the Creative Commons Attribution License.
Have therapeutics targeting autophagy shown efficacy in Parkinson’s disease models?
Scientists are exploring a number of autophagy-targeting approaches for PD drug development. Below are a few examples of experimental approaches in mouse models.
mTOR Inhibitors
The mTOR signaling pathway regulates cell growth, proliferation, and survival. mTOR protein expression is upregulated in the brains of transgenic mice with alpha-synuclein aggregates. Rapamycin, an mTOR inhibitor, is shown to promote autophagy in PD mouse models (Zhu, 2019). When Bai et al. (Bai, 2015) fed a rapamycin diet to ATG5 alpha-synuclein transgenic mice for 24 weeks, the mice had improved motor function and reduced loss of synaptophysin protein—a marker of synaptic integrity.
AMPK Activators
AMP-activated protein kinase (AMPK) signaling is involved in autophagy regulation during energy stress. AMPK activation has been shown to reduce alpha-synuclein aggregates and dopaminergic cell loss in PD models (Curry, 2018; Gao, 2019). However, emerging research is challenging the conventional understanding of AMPK’s role in autophagy. Recent biochemical analyses suggest that AMPK does not enhance autophagy initiation, but rather negatively regulates autophagy initiation under certain conditions (Kim, 2024). While AMPK remains a potential therapeutic target in PD, these findings highlight its context-dependent role in autophagy and the need for further investigation.
Transcription factor EB
Transcription factor EB (TFEB) is a regulator of lysosome biogenesis and autophagy. Overexpression of TFEB in PD mouse models has shown to activate autophagy-lysosome pathway and rescue dopaminergic neurons from alpha-synuclein toxicity, suggesting that there may be a mechanistic link between alpha-synuclein toxicity and impaired TFEB function (Decressac, 2013; Zhuang, 2020; Song, 2021).
For more on the role of lysosome dysfunction in neurodegenerative diseases, see: Lysosome Dysfunction in Microglia & Astrocytes
Our team would be happy to answer any questions about autophagy in Parkinson's disease or provide information about the PD models we use for therapeutic efficacy studies.
Discover more about our Parkinson's Disease Models
Related Content
Up-to-date information on autophagy in Parkinson's disease and best practices related to the evaluation of therapeutic agents in animal models of neurodegenerative diseases.
Impaired Microglia Autophagy in Neurodegenerative Diseases
How impaired microglia autophagy contributes to the progression of neurodegenerative diseases.
Autophagy & Neurodegenerative Diseases
An overview of how cellular autophagy plays a role in brain health and neurodegeneration.
Autophagy and Transcription Factor EB (TFEB)
An overview of Transcription Factor EB (TFEB) and its role in autophagy and neurodegenerative diseases.
Lysosome Dysfunction in Microglia & Astrocytes
An overview of lysosomal dysfunction in microglia & astrocytes, and its role in neurodegenerative diseases.