Anglade, P., Vyas, S., Javoy-Agid, F., Herrero, M.T., Michel, P.P., Marquez, J., Mouatt-Prigent, A., Ruberg, M., Hirsch, E.C., Agid, Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol., 12: 25–31, 1997; doi: 10.14670/HH-12.25. PMID: 9046040.
Bai, X., Wey, M.C.-Y., Fernandez, E., Hart, M.J., Gelfond, J., Bokov, A.F., Rani, S., Strong, R. Rapamycin improves motor function, reduces 4-hydroxynonenal adducted protein in brain, and attenuates synaptic injury in a mouse model of synucleinopathy. Pathobiol. Aging Aging Relat. Dis., 5: 28743, 2015; doi: 10.3402/pba.v5.28743
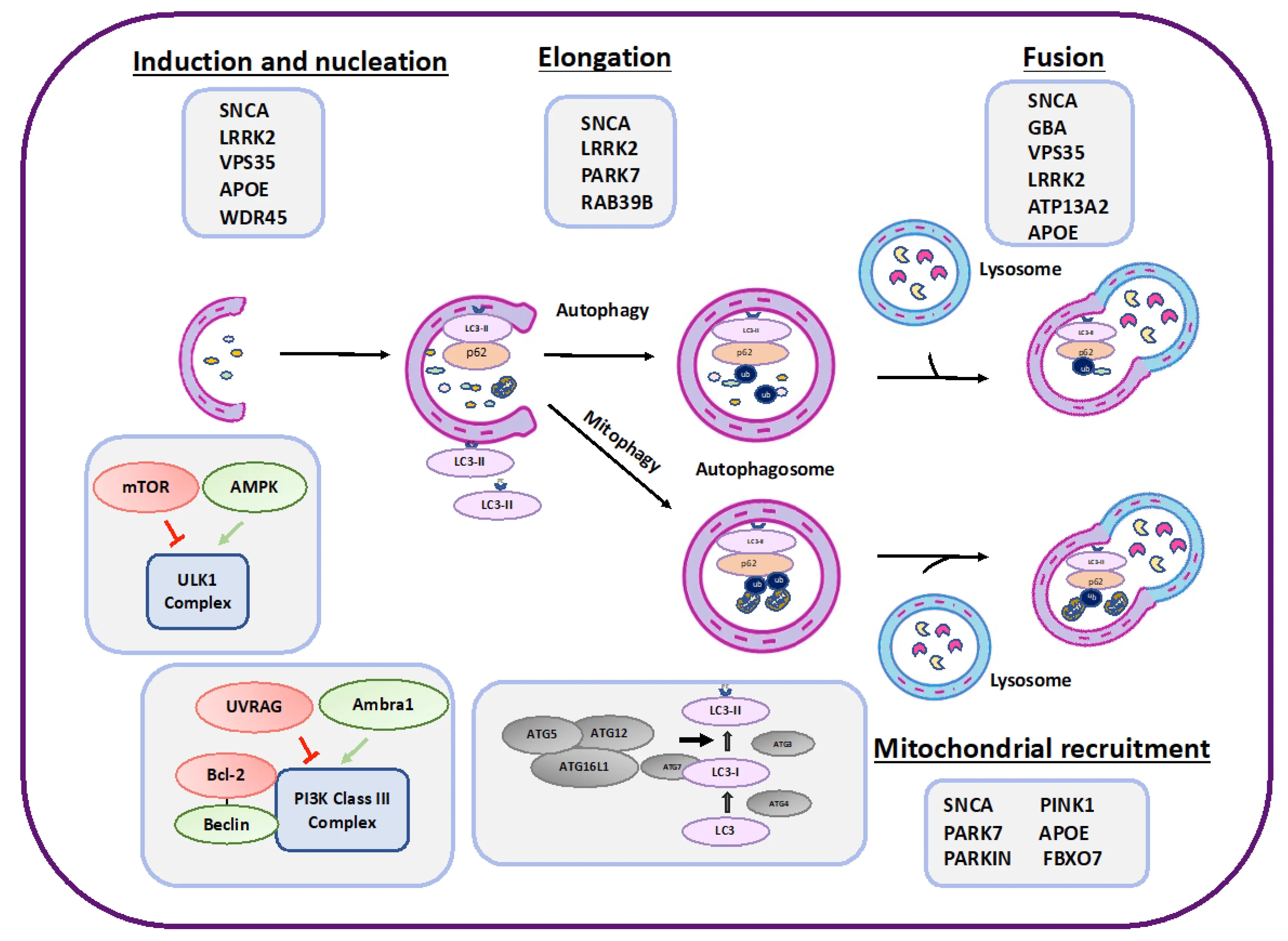
Beilina, A., Cookson, M.R. Genes associated with Parkinson’s disease: Regulation of autophagy and beyond. J. Neurochem., 139: 91–107, 2015; doi: 10.1111/jnc.13266
Bolam, J.P., Pissadaki, E.K. Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov. Disord., 27: 1478–1483, 2012; doi: 10.1002/mds.25135
Curry, D.W., Stutz, B., Andrews, Z.B., Elsworth, J.D. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. J. Parkinsons Dis., 8: 161–181, 2018; doi: 10.3233/JPD-171296
Decressac, M., Mattsson, B., Weikop, P., Lundblad, M., Jakobsson, J., Björklund, A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl. Acad. Sci. U.S.A., 110: E1817–E1826, 2013; doi: 10.1073/pnas.1305623110
Frake, R.A., Ricketts, T., Menzies, F.M., Rubinsztein, D.C. Autophagy and neurodegeneration. J. Clin. Invest., 125: 65–74, 2015; doi: 10.1172/JCI73944
Friedman, L.G., Lachenmayer, M.L., Wang, J., He, L., Poulose, S.M., Komatsu, M., Holstein, G.R., Yue, Z. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of α-synuclein and LRRK2 in the brain. J. Neurosci., 32: 7585–7593, 2012; doi: 10.1523/JNEUROSCI.5809-11.2012
Gao, J., Perera, G., Bhadbhade, M., Halliday, G.M., Dzamko, N. Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells. J. Biol. Chem., 294: 14241–14256, 2019; doi: 10.1074/jbc.RA119.008733
Han, R., Wang, Q., Xiong, X., Chen, X., Tu, Z., Li, B., Zhang, F., Chen, C., Pan, M., Xu, T., Chen, L., Wang, Z., Liu, Y., He, D., Guo, X., He, F., Wu, P., Yin, P., Liu, Y., Yang, W. Deficiency of parkin causes neurodegeneration and accumulation of pathological α-synuclein in monkey models. J. Clin. Invest., 134: 20, 2024; doi: 10.1172/JCI179633
Hernandez, D., Torres, C.A., Setlik, W., Cebrián, C., Mosharov, E.V., Tang, G., Cheng, H.-C., Kholodilov, N., Yarygina, O., Burke, R.E., Gershon, M., Sulzer, D. Regulation of presynaptic neurotransmission by macroautophagy. Neuron, 74: 277–284, 2012; doi: 10.1016/j.neuron.2012.02.020
Hou, X., Watzlawik, J.O., Fiesel, F.C., & Springer, W. Autophagy in Parkinson’s disease. J. Mol. Biol., 432: 2651–2672, 2020; doi: 10.1016/j.jmb.2020.01.037
Hunn, B.H.M., Vingill, S., Threlfell, S., Alegre-Abarrategui, J., Magdelyns, M., Deltheil, T., Bengoa-Vergniory, N., Oliver, P.L., Cioroch, M., Doig, N.M., Bannerman, D.M., Cragg, S.J., & Wade-Martins, R. Impairment of Macroautophagy in Dopamine Neurons Has Opposing Effects on Parkinsonian Pathology and Behavior. Cell Rep., 29: 920–931.e7, 2019; doi: 10.1016/j.celrep.2019.09.029
Kim, D.-H. Contrasting views on the role of AMPK in autophagy. BioEssays, 46: 2300211, 2024; doi: 10.1002/bies.202300211
Kinnart, I., Manders, L., Heyninck, T., Imberechts, D., Praschberger, R., Schoovaerts, N., Verfaillie, C., Verstreken, P., & Vandenberghe, W. Elevated α-synuclein levels inhibit mitophagic flux. NPJ Parkinsons Dis., 10: 1–15, 2024; doi: 10.1038/s41531-024-00696-0
Krzystek, T.J., Banerjee, R., Thurston, L., Huang, J., Swinter, K., Rahman, S.N., Falzone, T.L., & Gunawardena, S. Differential mitochondrial roles for α-synuclein in DRP1-dependent fission and PINK1/Parkin-mediated oxidation. Cell Death Dis., 12: 1–16, 2021; doi: 10.1038/s41419-021-04046-3
Moon, H.E., & Paek, S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol., 24: 103–116, 2015; doi: 10.5607/en.2015.24.2.103
Moors, T., Paciotti, S., Chiasserini, D., Calabresi, P., Parnetti, L., Beccari, T., & van de Berg, W.D.J. Lysosomal Dysfunction and α-Synuclein Aggregation in Parkinson’s Disease: Diagnostic Links. Mov. Disord., 31: 791–801, 2016; doi: 10.1002/mds.26562
Nechushtai, L., Frenkel, D., & Pinkas-Kramarski, R. Autophagy in Parkinson’s Disease. Biomolecules, 13, 1435, 2023; doi: 10.3390/biom13101435
Negi, S., Khurana, N., & Duggal, N. The misfolding mystery: α-synuclein and the pathogenesis of Parkinson’s disease. Neurochem. Int., 177: 105760, 2024; doi: 10.1016/j.neuint.2024.105760
Nguyen, T.T., Kim, Y.J., Lai, T.T., Nguyen, P.T., Koh, Y.H., Nguyen, L.T.N., Ma, H.-I., & Kim, Y.E. PTEN-Induced Putative Kinase 1 Dysfunction Accelerates Synucleinopathy. J. Parkinsons Dis., 12: 1201–1217, 2022; doi: 10.3233/JPD-213065
Pupyshev, A.B., Tikhonova, M.A., Akopyan, A.A., Tenditnik, M.V., Dubrovina, N.I., & Korolenko, T.A. Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson’s disease. Pharmacol. Biochem. Behav., 177: 1–11, 2019; doi: 10.1016/j.pbb.2018.12.005
Ren, Y.-H., Niu, X.-Y., Huang, H.-J., Hao, X.-D., Wang, P.-X., Chi, Y.-L., Ding, Y.-Q., & Liao, M. Dopamine neuron loss by selective deletion of autophagy-related gene 5 is not exacerbated by MPTP toxicity in midbrain. Neurosci. Lett., 675: 140–144, 2018; doi: 10.1016/j.neulet.2017.11.037
Sato, S., Uchihara, T., Fukuda, T., Noda, S., Kondo, H., Saiki, S., Komatsu, M., Uchiyama, Y., Tanaka, K., & Hattori, N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep., 8: 2813, 2018; doi: 10.1038/s41598-018-21325-w
Song, J.-X., Liu, J., Jiang, Y., Wang, Z.-Y., & Li, M. Transcription factor EB: An emerging drug target for neurodegenerative disorders. Drug Discov. Today, 26: 164–172, 2021; doi: 10.1016/j.drudis.2020.10.013
Tong, Y., Yamaguchi, H., Giaime, E., Boyle, S., Kopan, R., Kelleher, R.J., & Shen, J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. U.S.A., 107: 9879–9884, 2010; doi: 10.1073/pnas.1004676107
Vidal, R.L., Matus, S., Bargsted, L., & Hetz, C. Targeting autophagy in neurodegenerative diseases. Trends Pharmacol. Sci., 35: 583–591, 2014; doi: 10.1016/j.tips.2014.09.002
Vidović, M., & Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells, 11: 1732, 2022; doi: 10.3390/cells11111732
Vogiatzi, T., Xilouri, M., Vekrellis, K., & Stefanis, L. Wild Type α-Synuclein Is Degraded by Chaperone-mediated Autophagy and Macroautophagy in Neuronal Cells. J. Biol. Chem., 283: 23542–23556, 2008; doi: 10.1074/jbc.M801992200
Webb, J.L., Ravikumar, B., Atkins, J., Skepper, J.N., & Rubinsztein, D.C. Α-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem., 278, 25009–25013, 2003; doi: 10.1074/jbc.M300227200
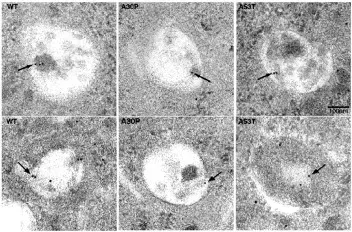
Winslow, A.R., Chen, C.-W., Corrochano, S., Acevedo-Arozena, A., Gordon, D.E., Peden, A.A., Lichtenberg, M., Menzies, F.M., Ravikumar, B., Imarisio, S., Brown, S., O’Kane, C.J., & Rubinsztein, D.C. α-Synuclein impairs macroautophagy: Implications for Parkinson’s disease. J. Cell Biol., 190: 1023–1037, 2010; doi: 10.1083/jcb.201003122
Wu, G., Wang, X., Feng, X., Zhang, A., Li, J., Gu, K., Huang, J., Pang, S., Dong, H., Gao, H., & Yan, B. Altered expression of autophagic genes in the peripheral leukocytes of patients with sporadic Parkinson’s disease. Brain Res., 1394: 105–111, 2011; doi: 10.1016/j.brainres.2011.04.013
Xilouri, M., Brekk, O.R., & Stefanis, L. Autophagy and alpha-synuclein: Relevance to Parkinson’s disease and related synucleopathies. Mov. Disord., 31: 178–192, 2016; doi: 10.1002/mds.26477
Yang, Y., Coleman, M., Zhang, L., Zheng, X., & Yue, Z. Autophagy in axonal and dendritic degeneration. Trends Neurosci., 36: 418–428, 2013; doi: 10.1016/j.tins.2013.04.001
Zhang, C., Chen, S., Li, X., Xu, Q., Lin, Y., Lin, F., Yuan, M., Zi, Y., & Cai, J. Progress in Parkinson’s disease animal models of genetic defects: Characteristics and application. Biomed. Pharmacother., 155: 113768, 2022; doi: 10.1016/j.biopha.2022.113768
Zhu, Z., Yang, C., Iyaswamy, A., Krishnamoorthi, S., Sreenivasmurthy, S.G., Liu, J., Wang, Z., Tong, B.C.-K., Song, J., Lu, J., Cheung, K.-H., & Li, M. Balancing mTOR signaling and autophagy in the treatment of Parkinson’s disease. Int. J. Mol. Sci., 20: 728, 2019; doi: 10.3390/ijms20030728
Zhuang, X.-X., Wang, S.-F., Tan, Y., Song, J.-X., Zhu, Z., Wang, Z.-Y., Wu, M.-Y., Cai, C.-Z., Huang, Z.-J., Tan, J.-Q., Su, H.-X., Li, M., & Lu, J.-H. Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson’s disease models. Cell Death Dis., 11: 1–18, 2020; doi: 10.1038/s41419-020-2322-6