Why measure neurofilament light in animal models of Parkinson's disease?
Neurofilament light (NfL; NF-L) is the light chain member of a family of intermediate filament proteins that localize to the axonal cytoplasm of neurons. When the axon is injured or degenerates, NfL is released into the extracellular space and can subsequently be measured in the cerebrospinal fluid (CSF) and blood (plasma, serum). As such, NfL levels serve as a fluid biomarker of axonal injury and/or neurodegeneration.
Our group has demonstrated a strong correlation between plasma & CSF NfL levels and whole brain volumes measured by in vivo magnetic resonance imaging (MRI) in an α-synuclein preformed fibril (PFF) seeding & spreading mouse model of Parkinson's disease. This analysis indicates the utility of this fluid biomarker to reflect brain atrophy. Note that NfL levels in biological fluids reflect aggregate neuronal damage across the entire nervous system without neuroanatomical specificity. On the other hand, MRI can measure regional changes in specific structures (e.g. distinct cortical and subcortical regions, spinal cord, etc.), including alterations of not only volume but also cortical thickness and spinal cord cross-sectional area. As such, blood & CSF NfL levels and structural MRI measures should be considered complementary modalities.
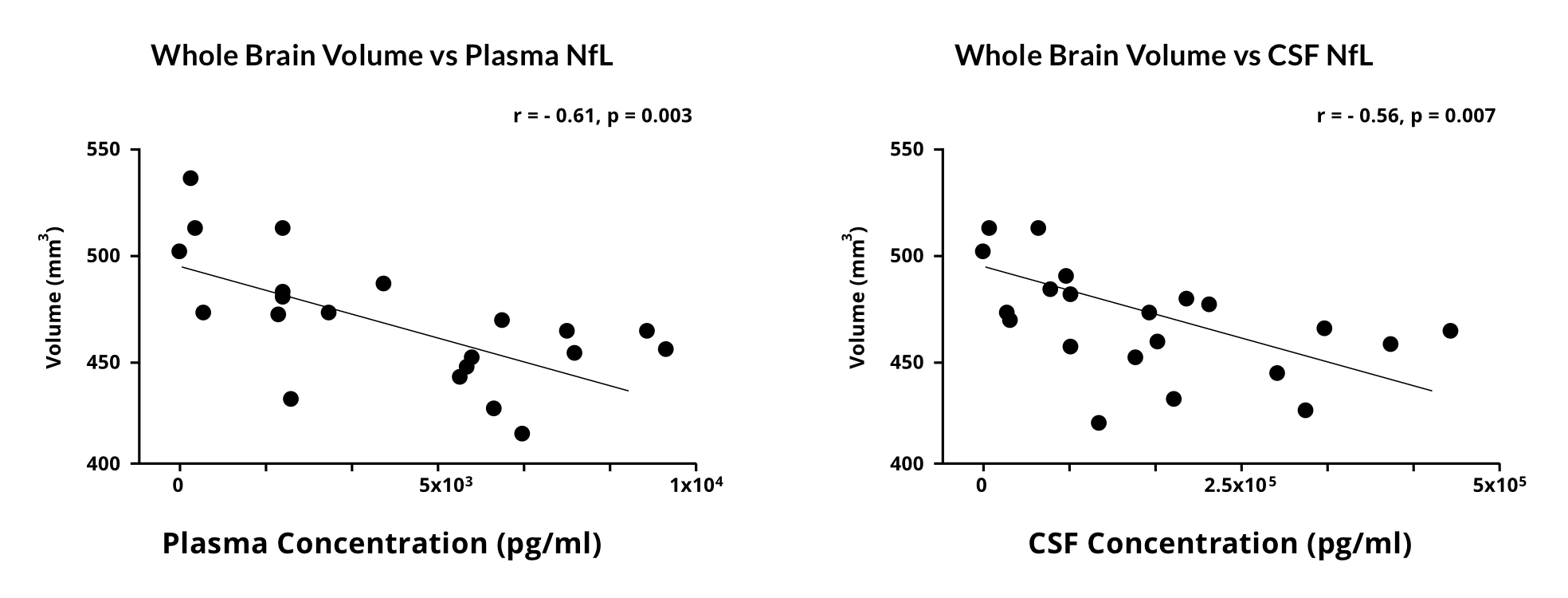
Correlations between plasma & CSF NfL levels and whole brain volumes measured by in vivo MRI in an α-synuclein mouse model of Parkinson's disease.
NfL levels can be measured at multiple time points during a preclinical therapeutic efficacy study, thereby allowing for a longitudinal assessment in the same animal. Multiple blood collections during the study can be easily performed. At Biospective, we also have the ability to collect multiple in-life CSF samples, thereby allowing for a comprehensive analysis of the temporal profile of fluid NfL levels to monitor disease progression and the effect of therapeutic intervention in animal models of Parkinson's disease.
How is neurofilament light measured in blood and CSF samples?
NfL is typically assessed in fluid samples using either an enzyme-linked immunosorbent assay (ELISA) or the ultra-sensitive single molecular array (Simoa) assay. The Simoa assay, as detailed in its original publication, surpasses ELISA's sensitivity by more than 1,000 times. Simoa represents an advanced single-molecule protein detection technology employing antibody-modified paramagnetic beads at exceptionally high concentrations relative to the very low concentrations of the target analyte. This setup ensures that each bead will capture, at most, one immune complex. Subsequently, the target analyte is labeled with a second targeted antibody complexed to a detection system, such as Streptavidin-β-Galactosidase (SβG), or multiple biotinylated detection reagents for multiplex analysis. Upon the introduction of a substrate, the detection reagent produces a fluorescent product. The crucial aspect of Simoa's sensitivity lies in the concentration of the resulting fluorescence intensity from an individual magnetic bead, confined within an oil covered microwell that accommodates only one bead. Fluorescent light captured from individual wells by a charge-coupled device (CCD) is utilized to acquire the signals. Through the calculation of the proportion of wells generating a signal from the array, the concentration of target proteins can be quantified.
The high sensitivity and low volume requirements of the Simoa assay make it an ideal tool for the analysis of mouse CSF and blood (plasma or serum) samples. CSF can only be collected in very small quantities, and NfL concentrations in blood are very low. This highly sensitive method can detect NfL levels as low as femtogram (fg/mL) levels.
Overview of the process for performing the collection and Simoa analysis of NfL concentrations from mouse biological fluids.
Recent advancements in Simoa have broadened its capabilities to include a multiplexing strategy, thereby facilitating the simultaneous evaluation of various disease-associated markers. Multiplexing is achieved through several techniques, such as using fluorescently-labeled paramagnetic beads in conjunction with targeted antibodies and detection reagents to generate a complex fluorescent signal capable of distinguishing multiple markers. For example, glial fibrillary acidic protein (GFAP), an intermediate filament found in astrocytes, can be measured concurrently with NfL. GFAP serves as a biomarker for astrogliosis, providing complementary insights alongside NfL, which serves as a biomarker for neuronal integrity. This multiplex Simoa technique has been successfully used to evaluate plasma levels of NFL, GFAP, UCHL1, and tau in Parkinson's disease patients.
In which animal models of Parkinson's disease have blood & CSF levels of neurofilament light been reported?
Our group routinely performs NfL measures in plasma and CSF from the α-synuclein preformed fibril (PFF) seeding & spreading mouse model. To generate this model, we inoculate recombinant human α-synuclein PFFs into specific regions of the mouse brain via stereotaxic injection to initiate the formation of α-synuclein aggregates which propagate along anatomically-connection pathways (so-called "prion-like spreading"). In order to generate a robust disease phenotype, we typically inject the fibrils unilaterally into the Anterior Olfactory Nucleus (AON) to generate a limbic system model or the Medial Forebrain Bundle (MFB) to produce a motor system model. We inject the fibrils into M83 transgenic mice which overexpress human α-synuclein with an A53T mutation under the mouse prion protein promoter (Prnp). We have demonstrated extensive neurodegeneration in this Parkinson's disease mouse model. We routinely observe highly elevated levels of NfL in both the plasma and CSF from these mice.
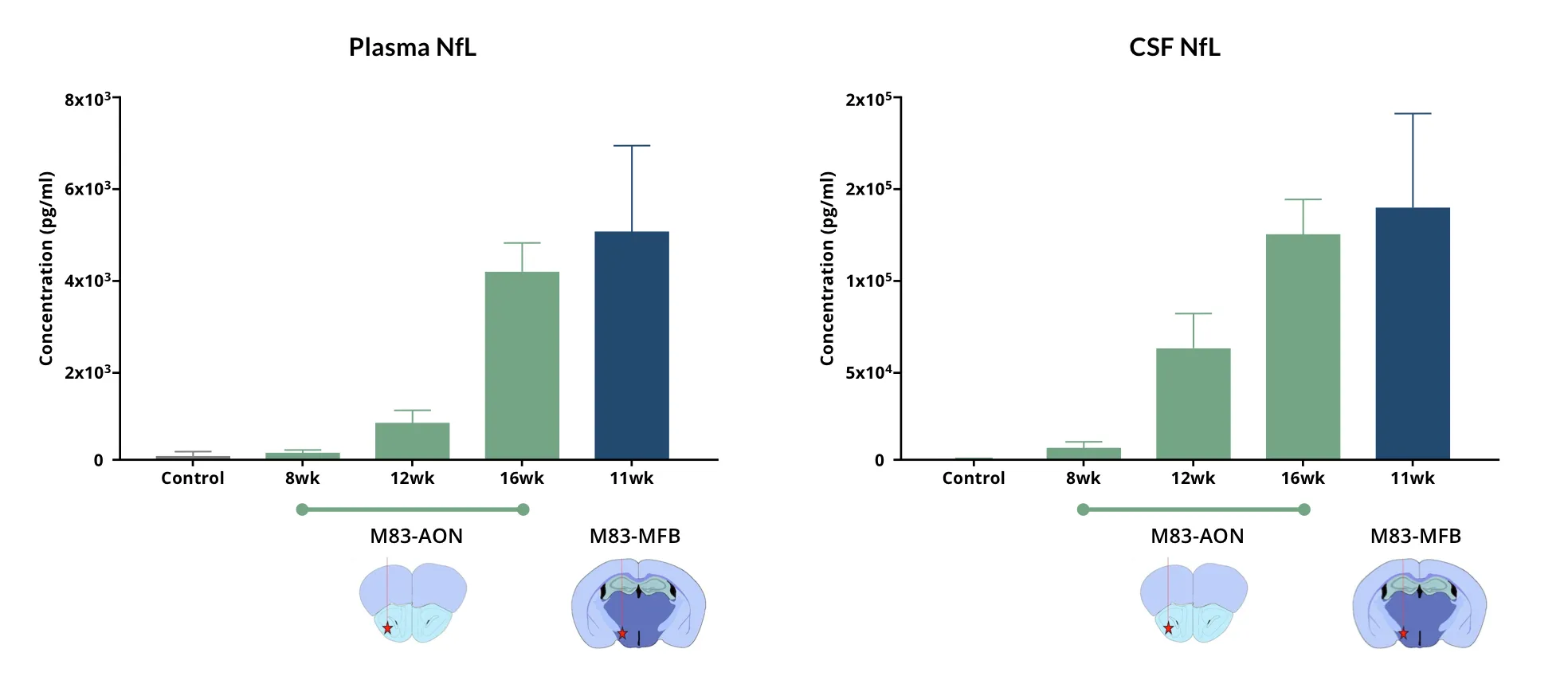
Highly elevated levels of NfL (mean ± s.d.) are found in the plasma and CSF of M83+/- transgenic mice injected with recombinant human α-synuclein PFFs into the AON and MFB.
Kasanga et al. found that serum NfL levels increased 40% after 6-hydroxydopamine (6-OHDA) lesioning in Sprague-Dawley rats compared to sham animals. This group also showed that moderate intensity aerobic exercise significantly reduced serum NfL levels in both sham and 6-OHDA groups, with a 42% reduction in the sham group and a 25% reduction in the 6-OHDA group.
Bacioglu and colleagues evaluated NfL levels in Thy1-hA53T-αS (A53T-αS) mice. In the A53T-αS mice, they found that a significant, greater than 10-fold increase in CSF NfL over non-transgenic controls was already observed in non-symptomatic 2- to 4-month-old mice. NfL levels subsequently increased and were 1,000-fold higher than age-matched non-transgenic control mice at 8 to 10 months-of-age. Plasma NfL levels did not show a significant increase at 2-4 months of age, but demonstrated greater than 100-fold increase at the symptomatic stage.
This group also assessed NfL levels in Thy1-hA30P-αS (A30P-αS) mice inoculated with brain extract derived from aged symptomatic A30P-αS transgenic mice. Measurements of NfL in both plasma and CSF from non-injected (control) A30P-αS mice demonstrated a robust age-related increase in NfL levels similar to that the A53T-αS mice at 18–22 months-of-age. However, injection of the A30P-αS mice with transgenic brain extract shifted this increase to 7–8 months-of-age. The authors, therefore, suggested a mechanistic link between the α-synuclein pathology and fluid NfL levels.
Loeffler et al. evaluated NfL levels in the Line 61 alpha-synuclein transgenic model. Plasma from male Line 61 mice was analyzed for NfL levels at 3, 6, 9, and 12 months-of-age. They found only a minor increase of NfL levels compared to non-transgenic littermates with a high degree of variability in older animals.
Clement et al. evaluated plasma and CSF levels in the MitoPark mouse model, but did not find any significant elevation compared to littermate controls.
Our team would be happy to answer any questions about Parkinson's Disease models or provide specific information about the models that we use for therapeutic efficacy studies.
Discover more about our Parkinson's Disease Models
Related Content
Up-to-date information on Parkinson's Disease and best practices related to the evaluation of therapeutic agents in PD animal models.
Autophagy, Parkinson's Disease, and Dopaminergic Neurons
An overview of how impaired autophagy can lead to pathologic changes and neurodegeneration in dopaminergic neurons in Parkinson’s disease.
Microglial Activation in an α-Synuclein PFF Mouse Model
We have quantified microglial activation, based on morphology, in an α-synuclein preformed fibril (PFF) seeding & spreading mouse model of Parkinson’s disease.
Brain Atrophy Analysis in Mouse Models of Neurodegeneration
Automated in vivo MRI-based quantitative brain atrophy measures (regional brain volumes and cortical thickness) from mouse models of ALS & Parkinson's disease.
AAV α-Synuclein Models for Parkinson's Disease Drug Development
Overview of adeno-associated virus (AAV) induced α-synuclein expression in mouse & rat models for use in preclinical studies of disease-modifying therapeutics.