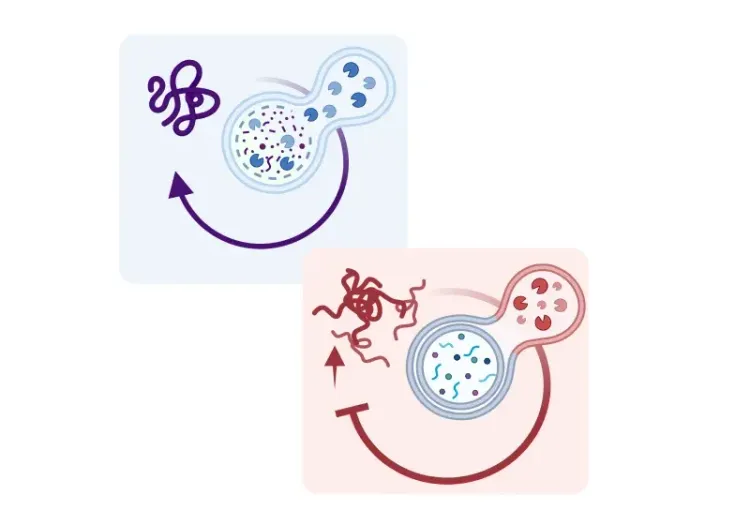
このリソースでは、以下の内容について説明します。
転写因子EB(TFEB)とは何ですか?
転写因子(TF)は、特定のDNA配列に結合することで遺伝子発現を調節するタンパク質のグループであり、DNAからRNAへの転写プロセスを阻害または促進することで、遺伝子を効果的に「オン」または「オフ」にします(Wang, 2015;Oksuz, 2023)。2009年に発見された転写因子EB(TFEB)は、小眼症関連転写因子/転写因子E(MiTF/TFE)ファミリーに属し、栄養素の感知、脂肪および糖代謝、オートファジー、細胞内小器官の形成と機能、酸化ストレス、微生物感染、ミトコンドリア損傷などの細胞ストレスに対する適応反応など、多様な役割を担っています(La Spina, 2021;Gebrie, 2023)。
当初は癌遺伝子として説明されていた TFEB は、現在ではオートファジー-リソソーム経路のマスター調節因子として認識されています。この経路は、タンパク質の凝集体、機能不全のオルガネラ、脂質、その他の構成成分などの細胞内物質をリソソームに輸送し、分解や新しい分子の合成を行うために不可欠です。TFEBは、オートファゴソーム形成、リソソーム生合成、リソソーム機能、脂質代謝、およびミトコンドリアの品質管理を担う遺伝子をアップレギュレートすることで、オートファジーを促進します(Sardiello, 2009;Napolitano, 2016;Cortes, 2020;Chen, 2024)。TFEBシグナル伝達は、飢餓、酸化ストレス、リソソームの損傷および機能不全、タンパク質の凝集、ミトコンドリアの損傷などのさまざまなストレス条件下で活性化されます(Wang, 2020;Franco-Juarez, 2022)。
中枢神経系(CNS)では、TFEBシグナル伝達はさらに多くの利点をもたらします。細胞内残屑の除去を促進し、シナプス機能を回復させ、神経細胞の代謝状態を調節することで、神経炎症を軽減し、神経可塑性を促進します(Wang, 2020;Gu, 2022;Matthews, 2023)。Matthews らによる最近の研究(Matthews, 2023)では、cTFEB;HSACre トランスジェニックマウスの骨格筋で TFEB を過剰発現させることで、これらの利点が実証されました。 この筋特異的 TFEB の過剰発現により、脳の炎症が軽減し、海馬の老化のバイオマーカーが改善し、シナプス伝達、神経可塑性、炎症反応を制御する遺伝子の発現が促進されました。
TFEBの活性を可視化し定量化するために、マウスや生細胞 を用いた生体内研究では、ルシフェラーゼやtdTomatoなどの分子レポーターを用いた生化学的アッセイと細胞イメージングを組み合わせることがよくあります。TFEBの細胞質から核への移行はリン酸化によって制御されているため、ウェスタンブロットは、リン酸化型(活性型)と非リン酸化型(不活性型)を区別するための重要な技術です(Brunialti, 2024)。
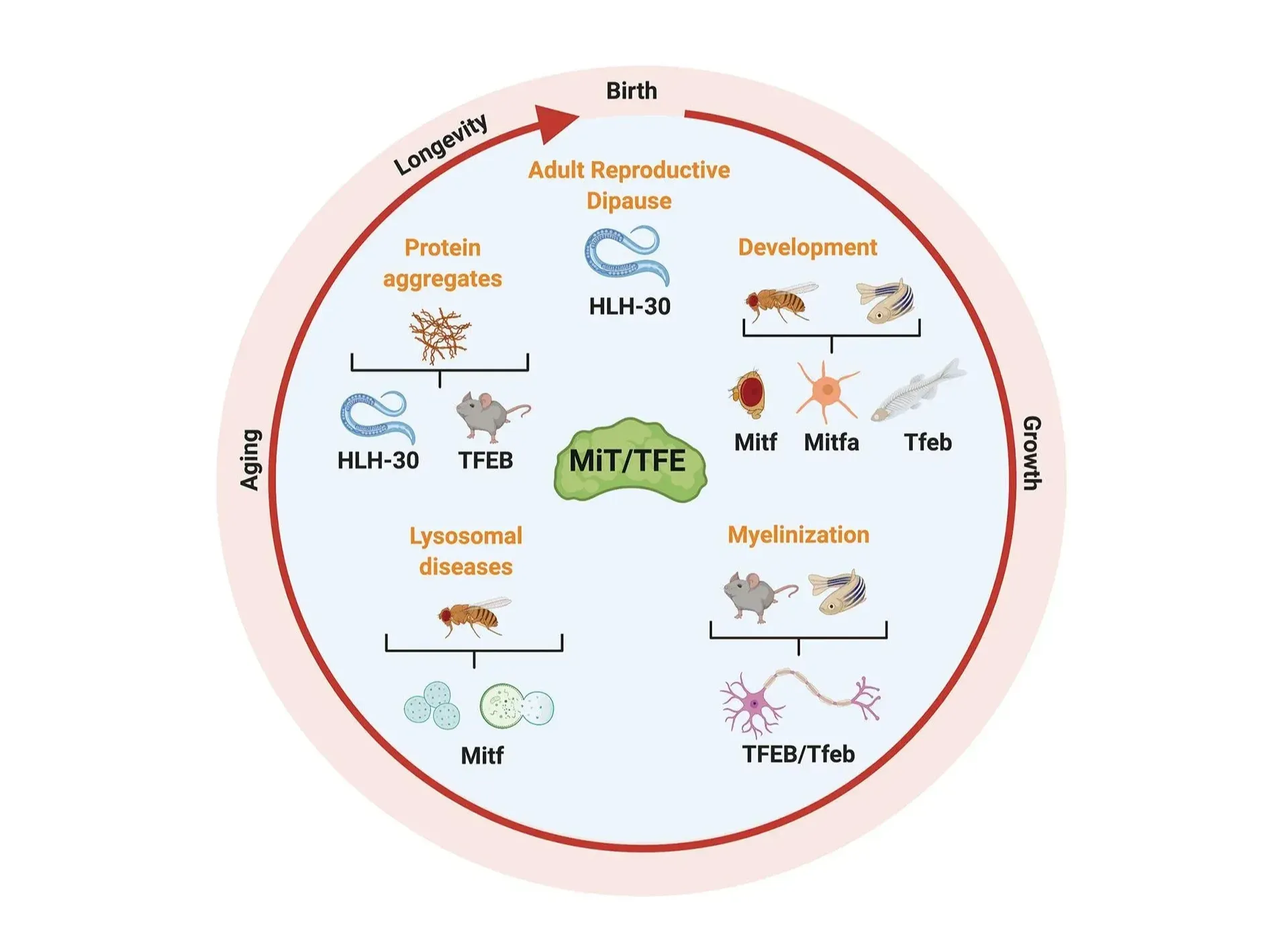
MiT/TFEファミリーのメンバーは、発生、寿命、ストレス応答など、さまざまな生物学的プロセスにおいて重要な役割を果たしています。図とキャプションは、La Spina et al. (La Spina, 2021) のクリエイティブ・コモンズ表示ライセンスに基づき転載しています。
TFEBとオートファジーの関係とは?
オートファジーの障害は、アルツハイマー病(AD)、パーキンソン病(PD)、筋萎縮性側索硬化症(ALS)、ハンチントン病(HD)を含むいくつかの神経変性疾患の病理と密接に関連しています(Nah, 2015;Deng, 2017;Menzies, 2017)。オートファジーの主要調節因子として、TFEBはリソソームの形成と機能に関わる遺伝子をアップレギュレートします(Palmieri, 2011;Settembre, 2011;Song, 2021)。これらの遺伝子は、CLEAR(Coordinated Lysosomal Expression and Regulation)ネットワークの一部であり、プロモーターにCLEARモチーフと呼ばれる共通のDNA配列を持っています。TFEBは、この10塩基のE-box配列に直接結合し、オートファゴソームの形成とオートファゴソームとリソソームの融合を誘導します(Palmieri, 2011;Settembre, 2011;Chen, 2024)。
神経変性疾患におけるオートファジーの障害の役割に関する詳細なレビューについては、以下のリンクを参照してください:オートファジーと神経変性疾患
オートファジーのマスターレギュレーターとしての役割により、TFEBの機能不全は神経変性の促進因子として作用します(Martini-Stoica, 2017;Cortes, 2019;Song, 2021)。マウスおよび細胞モデルを用いた研究により、TFEBの過剰発現がタンパク質凝集体の除去を促進することが示されています(Decressac, 2013;Polito, 2014;Chauhan, 2015)。しかし、TFEBの細胞質内滞留によりCLEARネットワークの活性が低下または損なわれると、神経変性疾患の病態生理に寄与します(Reddy, 2016)。Tiribuzi ら(Tiribuzi, 2014 )は、アルツハイマー病における TFEB の機能と局在に著しい変化があることを報告しています。彼らは、アルツハイマー病患者のリンパ球と単球を分析し、いくつかのリソソーム酵素の発現が低下していることを明らかにしました。このことは、TFEB の機能不全が、病気の過程で観察されるリソソームの欠損と関連している可能性があることを示しています。
また、TFEBとオートファジーの関係は複雑であり、その活性はタンパク質間相互作用を含む複数のリンクを介して制御されていることも注目に値します。オートファジーの阻害因子であるmTORC1は、TFEBを直接リン酸化し、その活性化と核内移行を妨げます。mTORC1の活性が低下または阻害されると、TFEBのようなタンパク質のリン酸化はもはや起こりません。このシナリオにより、TFEBは核へと移動し、そこでCLEARプロモーターに結合し、オートファジーが誘発されます(Martina, 2012;Zhang, 2020; Franco-Juarez, 2022)。
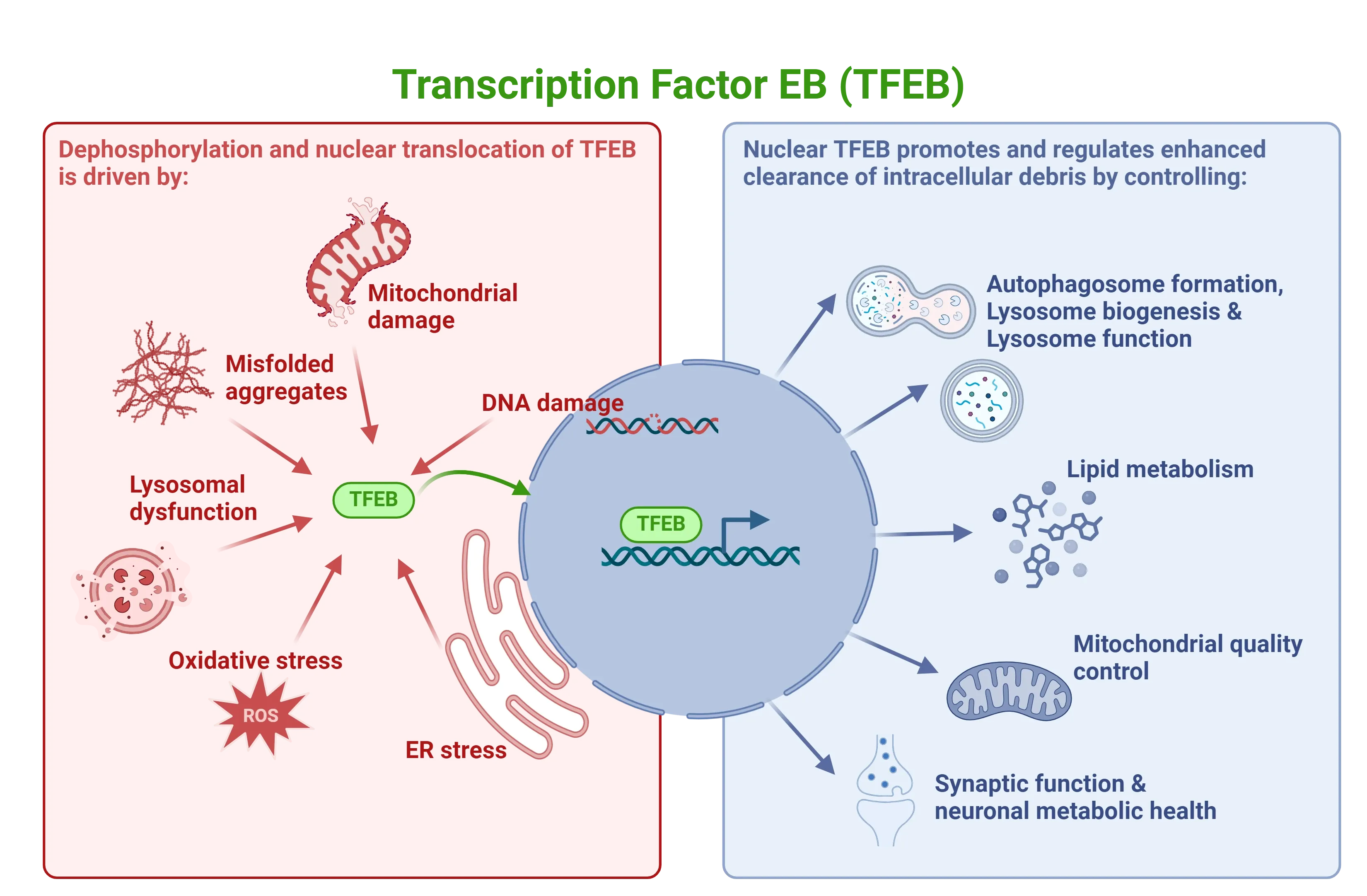
TFEBシグナル伝達は、酸化ストレス、リソソームの損傷や機能不全、タンパク質の凝集、ミトコンドリアの損傷など、さまざまなストレス条件下で活性化されます。核移行後、TFEBは、オートファゴソーム形成、リソソーム生合成、リソソーム機能、脂質代謝、ミトコンドリア品質管理、神経代謝の健康を司る遺伝子をアップレギュレートすることで、オートファジーを促進します。
TFEBを介したアプローチは、神経変性疾患の治療効果を示したことがありますか?
TFEBの活性化は、神経変性疾患の有望な治療戦略であり、複数の神経変性疾患モデルにおいて、オートファジーを確実に促進する能力があることが研究により示されています。
筋萎縮性側索硬化症(ALS)
Young 氏ら(Young, 2023)は、TFEB を活性化することが示されている低分子選択的 PIKfyve 阻害剤である AIT-101(INN:アピリモド、別名 LAM-002A)が、体重減少を減少させ、運動障害(後肢の抱え込み、後肢の麻痺、また、バイオスペクティブ社のALSモデルであるrNLS8のLow Doxバージョンでは、血漿および脳脊髄液中の神経フィラメント軽鎖(NfL)レベル、TDP-43凝集体(IHC法)、神経炎症(GFAP IHC法)の低下も伴って、TFEBを活性化し、体重減少を抑制し、運動障害(後肢の抱え込み、後肢の麻痺、
アルツハイマー病(AD)
家族性アルツハイマー病変異を5つ持つマウス(5xFAD)を用いた最近の研究(Chun ,2022) では、トラメチニブを経口投与することでアミロイドベータの沈着が減少し、認知機能が改善し、損傷した神経構造が修復されることが示されました。トラメチニブは、TFEBのリン酸化を阻害することで、TFEBが核へ移行することを可能にし、CLEAR遺伝子の発現を調節し、オートファジーを活性化することで、この疾患修飾を実現します。Polito 氏ら(Polito, 2014)は、アデノ随伴ウイルス(AAV)送達システムにより TFEB を活性化すると、rTg4510 マウスの脳における神経原線維変化(ADにおけるタウタンパク質の凝集体)が減少することも発見しました(Polito, 2014)。
パーキンソン病(PD)
mTORの阻害剤であるラパマイシンは、パーキンソン病のマウスモデルにおいてオートファジーを促進することが示されています。Bai ら(Bai, 2015)は、Atg5 αシヌクレイン遺伝子導入マウスに24週間ラパマイシンを投与したところ、運動機能が改善し、TFEBのオートファジー活性化により、パーキンソン病で一般的に減少するシナプトフィジンタンパク質の損失が減少することを実証しました。同様に、Decressac ら(Decressac, 2013)は、TFEB 媒介のオートファジーが、α-シヌクレイン毒性に関する生体内モデルにおけるA9 および A10 ドーパミン作動性神経細胞に対して神経保護効果を持つことを発見しました。
参照:オートファジー、パーキンソン病、およびドーパミン神経細胞
ハンチントン病(HD)
HDのトランスジェニックマウスモデルにおいて、恒見氏らは(Tsunemi, 2012)、ペルオキシソーム増殖因子活性化受容体ガンマ共役因子1-α(PGC-1α)の過剰発現が変異型ハンチンチン(mHTT)凝集体を除去し、HDマウスの神経機能を改善することを発見しました。PGC-1αは細胞のエネルギー代謝を制御し、TFEBを活性化することが知られており、TFEBはオートファジーを促進します。同様に、Vodicka ら(Vodicka, 2016)は、HD Q175/Q7マウスの線条体にTFEBを直接発現させると、オートファジーが促進され、mHTTレベルが低下することを示しました。これらの知見を総合すると、TFEBの活性化により、HDモデルにおける有毒タンパク質の凝集が緩和される可能性が示唆されます。
私たちのチームは、神経変性疾患におけるオートファジーと転写因子EB(TFEB)に関するご質問にお答えしたり、治療効果の研究に使用しているAD、PD、ALSのモデルに関する特定の情報を提供したりいたします。
神経変性疾患モデルについてさらに詳しく知る
関連コンテンツ
神経変性疾患におけるオートファジーとTranscription Factor EB (TFEB)に関する最新情報、および神経変性疾患の動物モデルにおける治療薬の評価に関するベストプラクティス。
神経変性疾患におけるミクログリアオートファジーの障害
ミクログリアのオートファジーの障害が神経変性疾患の進行にどのように関与しているか。
ミクログリアとアストロサイトのライソゾーム機能不全
ミクログリアとアストロサイトのライソゾーム機能不全の概要と、神経変性疾患におけるその役割。
オートファジーと神経変性疾患
細胞性オートファジーが脳の健康と神経変性において果たす役割についての概要。
オートファジー、パーキンソン病、ドーパミン作動性ニューロン
パーキンソン病における障害のあるオートファジーが、ドーパミン作動性ニューロンにおける病理学的変化と神経変性につながる仕組みの概要。