Akiyoshi, R., Wake, H., Kato, D., Horiuchi, H., Ono, R., Ikegami, A., Haruwaka, K., Omori, T., Tachibana, Y., Moorhouse, A.J., Nabekura, J. Microglia enhance synapse activity to promote local network synchronization. eneuro, 5: ENEURO.0088-18.2018, 2018; doi:10.1523/ENEURO.0088-18.2018.
Baalman, K., Marin, M.A., Ho, T.S.-Y., Godoy, M., Cherian, L., Robertson, C., Rasband, M.N. Axon initial segment–associated microglia. J. Neurosci., 35: 2283–2292, 2015; doi:10.1523/JNEUROSCI.3751-14.2015.
Barcia, C., Ros, C.M., Annese, V., Carrillo-de Sauvage, M.A., Ros-Bernal, F., Gómez, A., Yuste, J.E., Campuzano, C.M., De Pablos, V., Fernandez-Villalba, E., Herrero, M.T. ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Sci. Rep., 2: 809, 2012; doi:10.1038/srep00809.
Bechmann, I., Peter, S., Beyer, M., Gimsa, U., Nitsch, R. Presence of B7–2 (CD86) and lack of B7–1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo. FASEB J., 15: 1086–1088, 2001; doi:10.1096/fj.00-0563fje.
Bemiller, S.M., Maphis, N.M., Formica, S.V., Wilson, G.N., Miller, C.M., Xu, G., Kokiko-Cochran, O.N., Kim, K.-W., Jung, S., Cannon, J.L., Crish, S.D., Cardona, A.E., Lamb, B.T., Bhaskar, K. Genetically enhancing the expression of chemokine domain of CX3CL1 fails to prevent tau pathology in mouse models of tauopathy. J. Neuroinflammation, 15: 278, 2018; doi:10.1186/s12974-018-1310-6.
Bhaskar, K., Konerth, M., Kokiko-Cochran, O.N., Cardona, A., Ransohoff, R.M., Lamb, B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron, 68: 19–31, 2010; doi:10.1016/j.neuron.2010.08.023.
Brelstaff, J., Tolkovsky, A.M., Ghetti, B., Goedert, M., Spillantini, M.G. Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep., 24: 1939-1948.e4, 2018; doi:10.1016/j.celrep.2018.07.072.
Brettschneider, J., Toledo, J.B., Van Deerlin, V.M., Elman, L., McCluskey, L., Lee, V.M.-Y., Trojanowski, J.Q. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE, 7: e39216, 2012; doi:10.1371/journal.pone.0039216.
Brückner, G., Hausen, D., Härtig, W., Drlicek, M., Arendt, T., Brauer, K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience, 92: 791–805, 1999; doi:10.1016/S0306-4522(99)00071-8.
Chandel, N.S. Mitochondria as signaling organelles. BMC Biol., 12: 34, 2014; doi:10.1186/1741-7007-12-34.
Clark, K.C., Josephson, A., Benusa, S.D., Hartley, R.K., Baer, M., Thummala, S., Joslyn, M., Sword, B.A., Elford, H., Oh, U., Dilsizoglu‐Senol, A., Lubetzki, C., Davenne, M., DeVries, G.H., Dupree, J.L. Compromised axon initial segment integrity in EAE is preceded by microglial reactivity and contact. Glia, 64: 1190–1209, 2016; doi:10.1002/glia.22991.
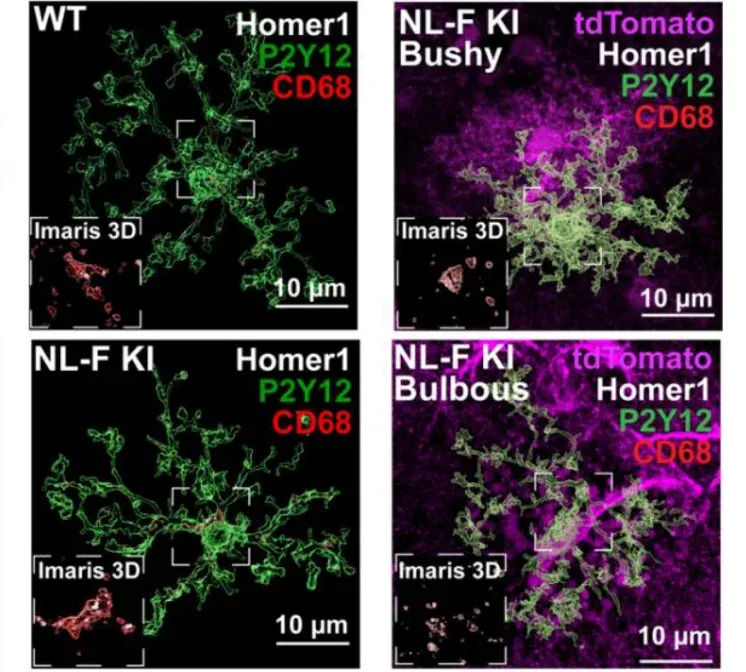
Crapser, J.D., Spangenberg, E.E., Barahona, R.A., Arreola, M.A., Hohsfield, L.A., Green, K.N. Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine, 58: 102919, 2020; doi:10.1016/j.ebiom.2020.102919.
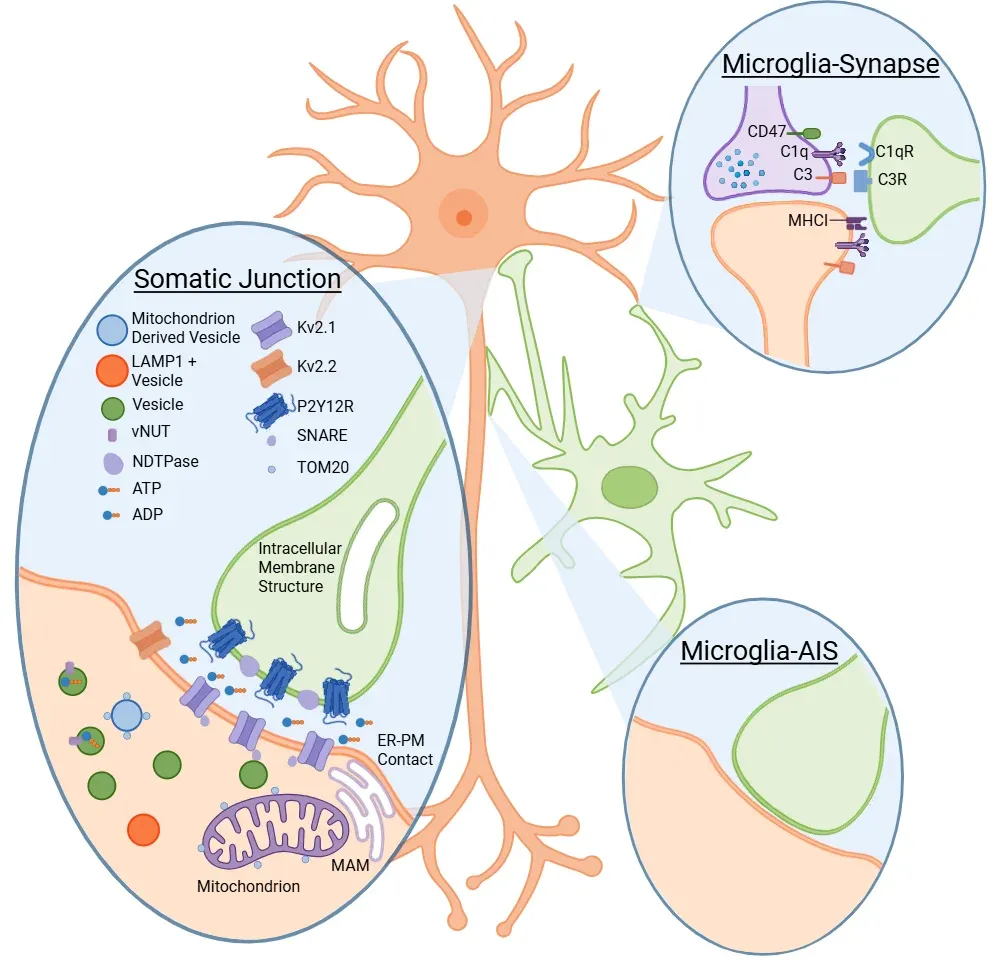
Cserép, C., Pósfai, B., Dénes, Á. Shaping neuronal fate: functional heterogeneity of direct microglia-neuron interactions. Neuron, 109: 222–240, 2021; doi:10.1016/j.neuron.2020.11.007.
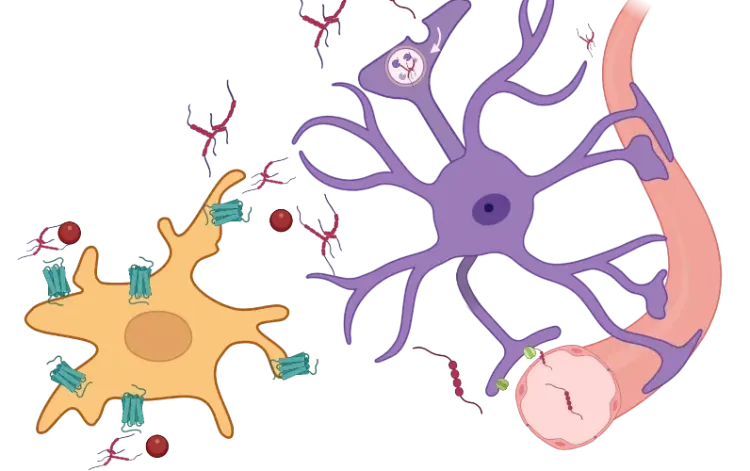
Cserép, C., Pósfai, B., Lénárt, N., Fekete, R., László, Z.I., Lele, Z., Orsolits, B., Molnár, G., Heindl, S., Schwarcz, A.D., Ujvári, K., Környei, Z., Tóth, K., Szabadits, E., Sperlágh, B., Baranyi, M., Csiba, L., Hortobágyi, T., … Dénes, Á. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science, 367: 528–537, 2020; doi:10.1126/science.aax6752.
Cserép, C., Schwarcz, A.D., Pósfai, B., László, Z.I., Kellermayer, A., Környei, Z., Kisfali, M., Nyerges, M., Lele, Z., Katona, I., Ádám Dénes. Microglial control of neuronal development via somatic purinergic junctions. Cell Rep., 40: 111369, 2022; doi:10.1016/j.celrep.2022.111369.
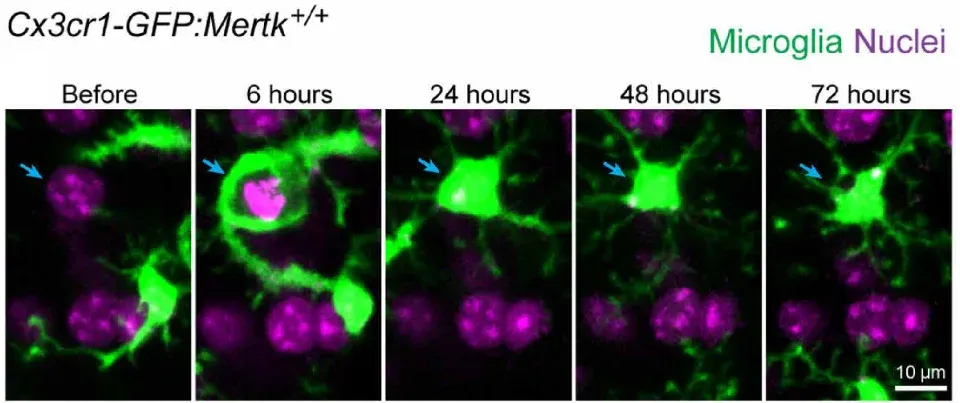
Damisah, E.C., Hill, R.A., Rai, A., Chen, F., Rothlin, C.V., Ghosh, S., Grutzendler, J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv., 6: eaba3239, 2020; doi:10.1126/sciadv.aba3239.
Dejanovic, B., Huntley, M.A., De Mazière, A., Meilandt, W.J., Wu, T., Srinivasan, K., Jiang, Z., Gandham, V., Friedman, B.A., Ngu, H., Foreman, O., Carano, R.A.D., Chih, B., Klumperman, J., Bakalarski, C., Hanson, J.E., Sheng, M. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron, 100: 1322-1336.e7, 2018; doi:10.1016/j.neuron.2018.10.014.
Fekete, R., Cserép, C., Lénárt, N., Tóth, K., Orsolits, B., Martinecz, B., Méhes, E., Szabó, B., Németh, V., Gönci, B., Sperlágh, B., Boldogkői, Z., Kittel, Á., Baranyi, M., Ferenczi, S., Kovács, K., Szalay, G., Rózsa, B., … Dénes, Á. Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol. (Berl.), 136: 461–482, 2018; doi:10.1007/s00401-018-1885-0.
Fracassi, A., Marcatti, M., Tumurbaatar, B., Woltjer, R., Moreno, S., Taglialatela, G. TREM2 ‐induced activation of microglia contributes to synaptic integrity in cognitively intact aged individuals with Alzheimer’s neuropathology. Brain Pathol., 33: e13108, 2023; doi:10.1111/bpa.13108.
Gao, C., Jiang, J., Tan, Y., Chen, S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct. Target. Ther., 8: 2023; doi:10.1038/s41392-023-01588-0.
Hall, C.N., Klein-Flugge, M.C., Howarth, C., Attwell, D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J. Neurosci., 32: 8940–8951, 2012; doi:10.1523/JNEUROSCI.0026-12.2012.
Hong, S., Beja-Glasser, V.F., Nfonoyim, B.M., Frouin, A., Li, S., Ramakrishnan, S., Merry, K.M., Shi, Q., Rosenthal, A., Barres, B.A., Lemere, C.A., Selkoe, D.J., Stevens, B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352: 712–716, 2016; doi:10.1126/science.aad8373.
Hoshiko, M., Arnoux, I., Avignone, E., Yamamoto, N., Audinat, E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J. Neurosci., 32: 15106–15111, 2012; doi:10.1523/JNEUROSCI.1167-12.2012.
Janda, E., Boi, L., Carta, A.R. Microglial phagocytosis and its regulation: a therapeutic target in Parkinson’s disease? Front. Mol. Neurosci., 11: 144, 2018; doi:10.3389/fnmol.2018.00144.
Kasahara, A., Scorrano, L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol., 24: 761–770, 2014; doi:10.1016/j.tcb.2014.08.005.
Kato, G., Inada, H., Wake, H., Akiyoshi, R., Miyamoto, A., Eto, K., Ishikawa, T., Moorhouse, A.J., Strassman, A.M., Nabekura, J. Microglial contact prevents excess depolarization and rescues neurons from excitotoxicity. eneuro, 3: ENEURO.0004-16.2016, 2016; doi:10.1523/ENEURO.0004-16.2016.
Lafrenaye, A.D., Todani, M., Walker, S.A., Povlishock, J.T. Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J. Neuroinflammation, 12: 186, 2015; doi:10.1186/s12974-015-0405-6.
Lall, D., Lorenzini, I., Mota, T.A., Bell, S., Mahan, T.E., Ulrich, J.D., Davtyan, H., Rexach, J.E., Muhammad, A.K.M.G., Shelest, O., Landeros, J., Vazquez, M., Kim, J., Ghaffari, L., O’Rourke, J.G., Geschwind, D.H., Blurton-Jones, M., Holtzman, D.M., … Baloh, R.H. C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation. Neuron, 109: 2275-2291.e8, 2021; doi:10.1016/j.neuron.2021.05.020.
Lee, S., Xu, G., Jay, T.R., Bhatta, S., Kim, K.-W., Jung, S., Landreth, G.E., Ransohoff, R.M., Lamb, B.T. Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J. Neurosci., 34: 12538–12546, 2014; doi:10.1523/JNEUROSCI.0853-14.2014.
Lloyd, A.F., Davies, C.L., Holloway, R.K., Labrak, Y., Ireland, G., Carradori, D., Dillenburg, A., Borger, E., Soong, D., Richardson, J.C., Kuhlmann, T., Williams, A., Pollard, J.W., Des Rieux, A., Priller, J., Miron, V.E. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci., 22: 1046–1052, 2019; doi:10.1038/s41593-019-0418-z.
Lui, H., Zhang, J., Makinson, S.R., Cahill, M.K., Kelley, K.W., Huang, H.-Y., Shang, Y., Oldham, M.C., Martens, L.H., Gao, F., Coppola, G., Sloan, S.A., Hsieh, C.L., Kim, C.C., Bigio, E.H., Weintraub, S., Mesulam, M.-M., Rademakers, R., … Huang, E.J. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell, 165: 921–935, 2016; doi:10.1016/j.cell.2016.04.001.
Makarava, N., Safadi, T., Bocharova, O., Mychko, O., Pandit, N.P., Molesworth, K., Eyo, U.B., Baskakov, I.V. Knockout of P2Y12 receptor facilitates microglia-neuron body-to-body interactions and accelerates prion disease. bioRxiv, 2025; doi:10.1101/2025.04.07.647619.
Maniatis, S., Äijö, T., Vickovic, S., Braine, C., Kang, K., Mollbrink, A., Fagegaltier, D., Andrusivová, Ž., Saarenpää, S., Saiz-Castro, G., Cuevas, M., Watters, A., Lundeberg, J., Bonneau, R., Phatnani, H. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science, 364: 89–93, 2019; doi:10.1126/science.aav9776.
Miyata, S., Nishimura, Y., Nakashima, T. Perineuronal nets protect against amyloid β-protein neurotoxicity in cultured cortical neurons. Brain Res., 1150: 200–206, 2007; doi:10.1016/j.brainres.2007.02.066.
Neumann, H., Kotter, M.R., Franklin, R.J.M. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain, 132: 288–295, 2009; doi:10.1093/brain/awn109.
Nonaka, S., Nakanishi, H. Microglial clearance of focal apoptotic synapses. Neurosci. Lett., 707: 134317, 2019; doi:10.1016/j.neulet.2019.134317.
Paolicelli, R.C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., Giustetto, M., Ferreira, T.A., Guiducci, E., Dumas, L., Ragozzino, D., Gross, C.T. Synaptic pruning by microglia is necessary for normal brain development. Science, 333: 1456–1458, 2011; doi:10.1126/science.1202529.
Parkhurst, C.N., Yang, G., Ninan, I., Savas, J.N., Yates, J.R., Lafaille, J.J., Hempstead, B.L., Littman, D.R., Gan, W.-B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155: 1596–1609, 2013; doi:10.1016/j.cell.2013.11.030.
Pfeiffer, T., Avignone, E., Nägerl, U.V. Induction of hippocampal long-term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines. Sci. Rep., 6: 32422, 2016; doi:10.1038/srep32422.
Puigdellívol, M., Milde, S., Vilalta, A., Cockram, T.O.J., Allendorf, D.H., Lee, J.Y., Dundee, J.M., Pampuščenko, K., Borutaite, V., Nuthall, H.N., Brelstaff, J.H., Spillantini, M.G., Brown, G.C. The microglial P2Y6 receptor mediates neuronal loss and memory deficits in neurodegeneration. Cell Rep., 37: 110148, 2021; doi:10.1016/j.celrep.2021.110148.
Rajendran, L., Paolicelli, R.C. Microglia-mediated synapse loss in Alzheimer’s Disease. J. Neurosci., 38: 2911–2919, 2018; doi:10.1523/JNEUROSCI.1136-17.2017.
Reichelt, A.C., Hare, D.J., Bussey, T.J., Saksida, L.M. Perineuronal nets: plasticity, protection, and therapeutic potential. Trends Neurosci., 42: 458–470, 2019; doi:10.1016/j.tins.2019.04.003.
Rogers, J.T., Morganti, J.M., Bachstetter, A.D., Hudson, C.E., Peters, M.M., Grimmig, B.A., Weeber, E.J., Bickford, P.C., Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci., 31: 16241–16250, 2011; doi:10.1523/JNEUROSCI.3667-11.2011.
Rugarli, E.I., Langer, T. Mitochondrial quality control: a matter of life and death for neurons: Mitochondrial quality control and neurodegeneration. EMBO J., 31: 1336–1349, 2012; doi:10.1038/emboj.2012.38.
Schafer, D.P., Lehrman, E.K., Kautzman, A.G., Koyama, R., Mardinly, A.R., Yamasaki, R., Ransohoff, R.M., Greenberg, M.E., Barres, B.A., Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74: 691–705, 2012; doi:10.1016/j.neuron.2012.03.026.
Shi, Q., Chowdhury, S., Ma, R., Le, K.X., Hong, S., Caldarone, B.J., Stevens, B., Lemere, C.A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med., 9: eaaf6295, 2017; doi:10.1126/scitranslmed.aaf6295.
Sierra, A., Encinas, J.M., Deudero, J.J.P., Chancey, J.H., Enikolopov, G., Overstreet-Wadiche, L.S., Tsirka, S.E., Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell, 7: 483–495, 2010; doi:10.1016/j.stem.2010.08.014.
Sokolova, D., Ghansah, S.A., Puletti, F., Georgiades, T., De Schepper, S., Zheng, Y., Crowley, G., Wu, L., Rueda-Carrasco, J., Koutsiouroumpa, A., Muckett, P., Freeman, O.J., Khakh, B.S., Hong, S. Astrocyte-derived MFG-E8 facilitates microglial synapse elimination in Alzheimer’s disease mouse models. bioRxiv, 2024; doi:10.1101/2024.08.31.606944.
Squarzoni, P., Oller, G., Hoeffel, G., Pont-Lezica, L., Rostaing, P., Low, D., Bessis, A., Ginhoux, F., Garel, S. Microglia modulate wiring of the embryonic forebrain. Cell Rep., 8: 1271–1279, 2014; doi:10.1016/j.celrep.2014.07.042.
Stevens, B., Allen, N.J., Vazquez, L.E., Howell, G.R., Christopherson, K.S., Nouri, N., Micheva, K.D., Mehalow, A.K., Huberman, A.D., Stafford, B., Sher, A., Litke, A.M., Lambris, J.D., Smith, S.J., John, S.W.M., Barres, B.A. The classical complement cascade mediates CNS synapse elimination. Cell, 131: 1164–1178, 2007; doi:10.1016/j.cell.2007.10.036.
Styr, B., Gonen, N., Zarhin, D., Ruggiero, A., Atsmon, R., Gazit, N., Braun, G., Frere, S., Vertkin, I., Shapira, I., Harel, M., Heim, L.R., Katsenelson, M., Rechnitz, O., Fadila, S., Derdikman, D., Rubinstein, M., Geiger, T., … Slutsky, I. Mitochondrial regulation of the hippocampal firing rate set point and seizure susceptibility. Neuron, 102: 1009-1024.e8, 2019; doi:10.1016/j.neuron.2019.03.045.
Suttkus, A., Holzer, M., Morawski, M., Arendt, T. The neuronal extracellular matrix restricts distribution and internalization of aggregated Tau-protein. Neuroscience, 313: 225–235, 2016; doi:10.1016/j.neuroscience.2015.11.040.
Szepesi, Z., Manouchehrian, O., Bachiller, S., Deierborg, T. Bidirectional microglia–neuron communication in health and disease. Front. Cell. Neurosci., 12: 323, 2018; doi:10.3389/fncel.2018.00323.
Tepikin, A.V. Mitochondrial junctions with cellular organelles: Ca2+ signalling perspective. Pflüg. Arch. - Eur. J. Physiol., 470: 1181–1192, 2018; doi:10.1007/s00424-018-2179-z.
Trapp, B.D., Wujek, J.R., Criste, G.A., Jalabi, W., Yin, X., Kidd, G.J., Stohlman, S., Ransohoff, R. Evidence for synaptic stripping by cortical microglia. Glia, 55: 360–368, 2007; doi:10.1002/glia.20462.
Tremblay, M.-È., Lowery, R.L., Majewska, A.K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol., 8: e1000527, 2010; doi:10.1371/journal.pbio.1000527.
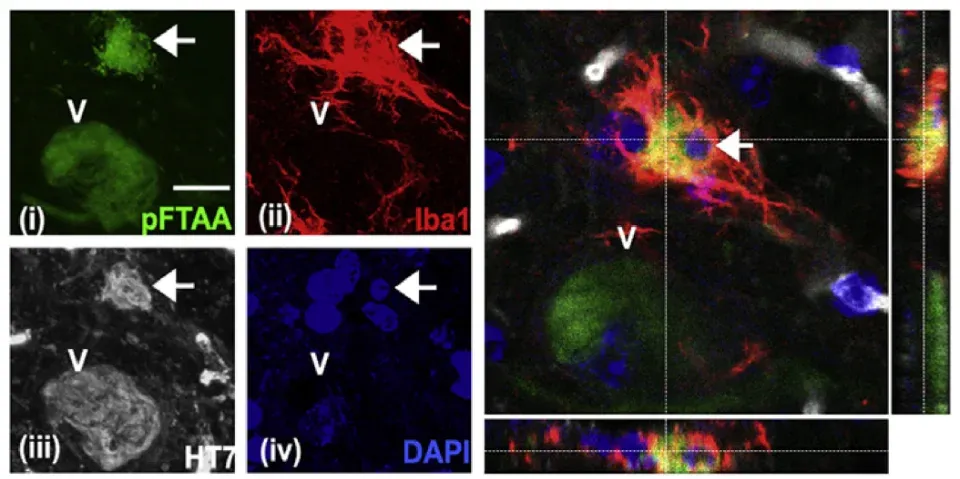
Vogels, T., Murgoci, A.-N., Hromádka, T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol. Commun., 7: 109, 2019; doi:10.1186/s40478-019-0754-y.
von Saucken, V.E., Jay, T.R., Landreth, G.E. The effect of amyloid on microglia-neuron interactions before plaque onset occurs independently of TREM2 in a mouse model of Alzheimer’s disease. Neurobiol. Dis., 145: 105072, 2020; doi:10.1016/j.nbd.2020.105072.
Wang, C., Xiong, M., Gratuze, M., Bao, X., Shi, Y., Andhey, P.S., Manis, M., Schroeder, C., Yin, Z., Madore, C., Butovsky, O., Artyomov, M., Ulrich, J.D., Holtzman, D.M. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron, 109: 1657-1674.e7, 2021; doi:10.1016/j.neuron.2021.03.024.
Wang, C., Yue, H., Hu, Z., Shen, Y., Ma, J., Li, J., Wang, X.-D., Wang, L., Sun, B., Shi, P., Wang, L., Gu, Y. Microglia mediate forgetting via complement-dependent synaptic elimination. Science, 367: 688–694, 2020; doi:10.1126/science.aaz2288.
Weinhard, L., Di Bartolomei, G., Bolasco, G., Machado, P., Schieber, N.L., Neniskyte, U., Exiga, M., Vadisiute, A., Raggioli, A., Schertel, A., Schwab, Y., Gross, C.T. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun., 9: 1228, 2018; doi:10.1038/s41467-018-03566-5.
Xie, M., Miller, A.S., Pallegar, P.N., Umpierre, A., Liang, Y., Wang, N., Zhang, S., Nagaraj, N.K., Fogarty, Z.C., Ghayal, N.B., Oskarsson, B., Zhao, S., Zheng, J., Qi, F., Nguyen, A., Dickson, D.W., Wu, L.-J. Rod-shaped microglia interact with neuronal dendrites to regulate cortical excitability in TDP-43 related neurodegeneration. bioRxiv, 2024; doi:10.1101/2024.06.30.601396.
Yamada, J., Hayashi, Y., Jinno, S., Wu, Z., Inoue, K., Kohsaka, S., Nakanishi, H. Reduced synaptic activity precedes synaptic stripping in vagal motoneurons after axotomy. Glia, 56: 1448–1462, 2008; doi:10.1002/glia.20711.
Zhan, Y., Paolicelli, R.C., Sforazzini, F., Weinhard, L., Bolasco, G., Pagani, F., Vyssotski, A.L., Bifone, A., Gozzi, A., Ragozzino, D., Gross, C.T. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci., 17: 400–406, 2014; doi:10.1038/nn.3641.
Zhang, J., Velmeshev, D., Hashimoto, K., Huang, Y.-H., Hofmann, J.W., Shi, X., Chen, J., Leidal, A.M., Dishart, J.G., Cahill, M.K., Kelley, K.W., Liddelow, S.A., Seeley, W.W., Miller, B.L., Walther, T.C., Farese, R.V., Taylor, J.P., Ullian, E.M., … Huang, E.J. Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency. Nature, 588: 459–465, 2020; doi:10.1038/s41586-020-2709-7.