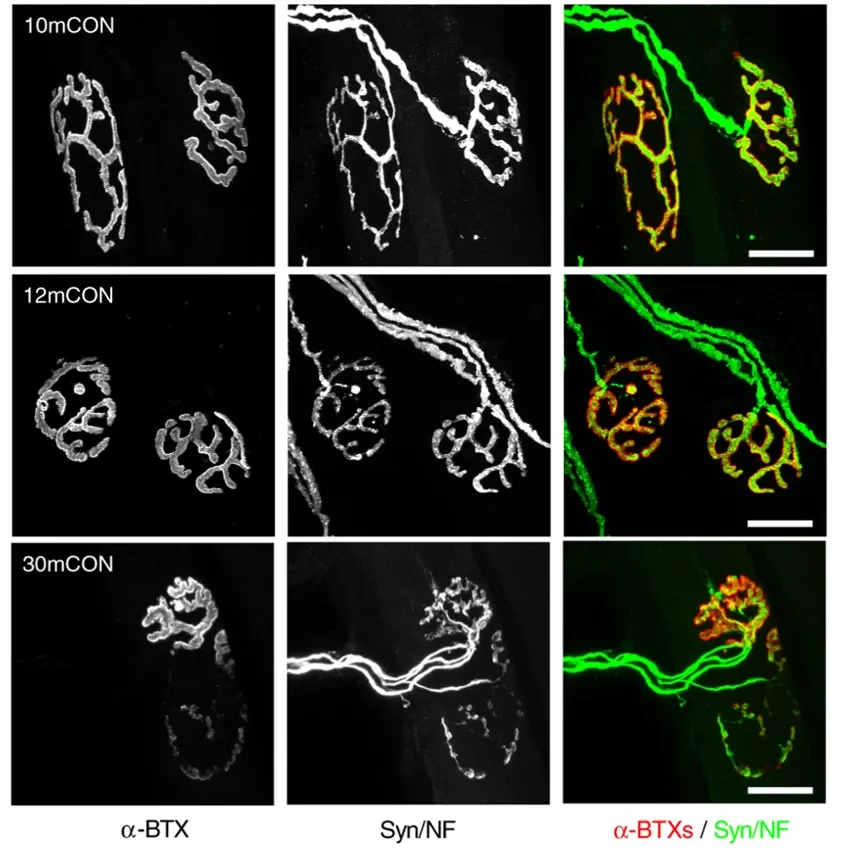
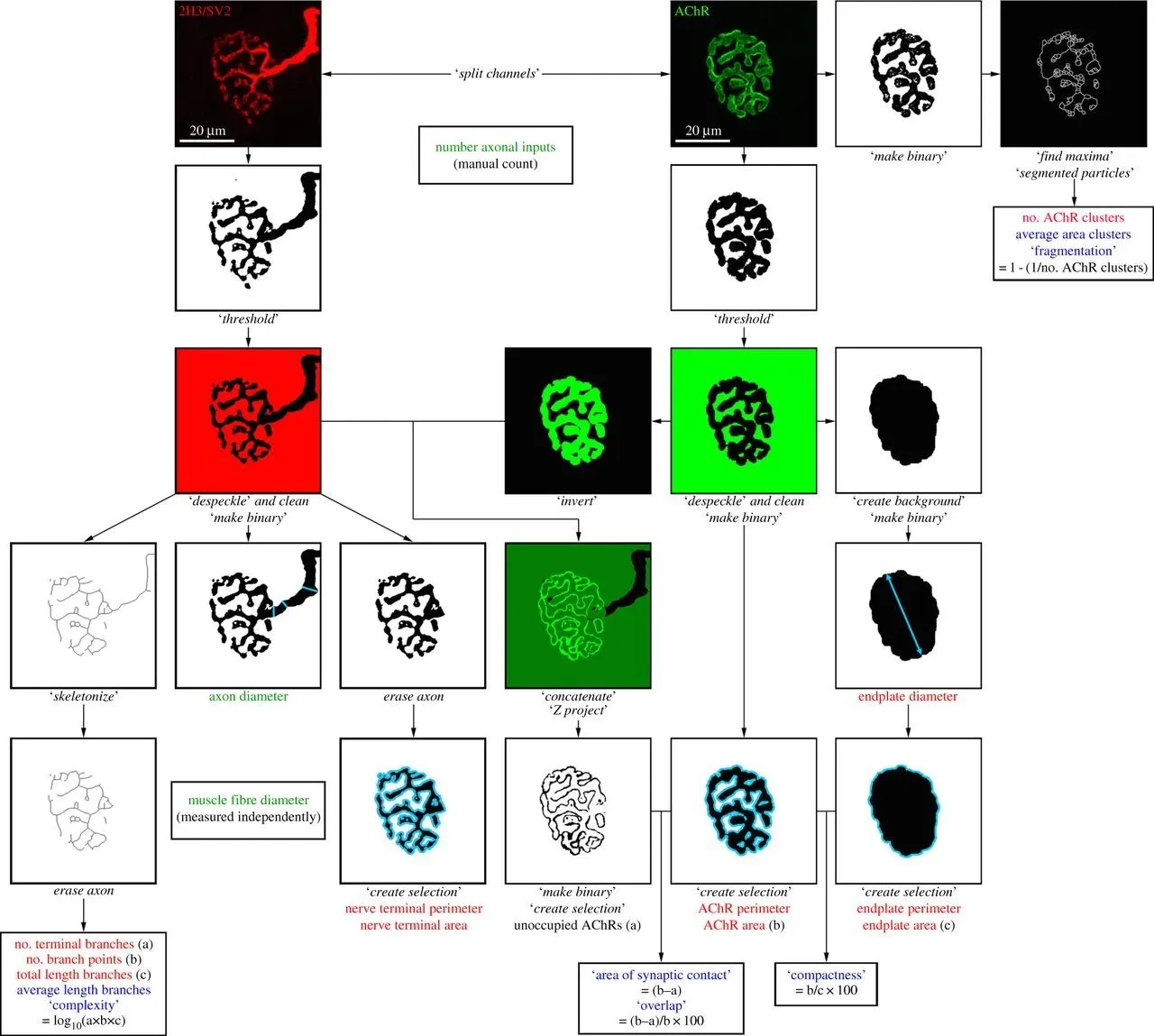
Alhindi, A., Boehm, I., Chaytow, H. Small junction, big problems: Neuromuscular junction pathology in mouse models of amyotrophic lateral sclerosis (ALS). J. Anat., 241: 1089–1107, 2022; doi: 10.1111/JOA.13463
Alhindi, A., Boehm, I., Forsythe, R.O., Miller, J., Skipworth, R.J.E., Simpson, H., Jones, R.A., Gillingwater, T.H. Terminal Schwann cells at the human neuromuscular junction. Brain Commun., 3: 2021; doi: 10.1093/BRAINCOMMS/FCAB081
Alhindi, A., Shand, M., Smith, H.L., Leite, A.S., Huang, Y.T., van der Hoorn, D., Ridgway, Z., Faller, K.M.E., Jones, R.A., Gillingwater, T.H., Chaytow, H. Neuromuscular junction denervation and terminal Schwann cell loss in the hTDP-43 overexpression mouse model of amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol., 49: e12925, 2023; doi: 10.1111/NAN.12925
Badawi, Y., Nishimune, H. Super-resolution microscopy for analyzing neuromuscular junctions and synapses. Neurosci. Lett., 715: 134644, 2020; doi: 10.1016/j.neulet.2019.134644
Boehm, I., Miller, J., Wishart, T.M., Wigmore, S.J., Skipworth, R.J.E., Jones, R.A., Gillingwater, T.H. Neuromuscular junctions are stable in patients with cancer cachexia. J. Clin. Invest., 130: 1461–1465, 2020; doi: 10.1172/JCI128411
Bruneteau, G., Bauché, S., De Aguilar, J.L.G., Brochier, G., Mandjee, N., Tanguy, M.L., Hussain, G., Behin, A., Khiami, F., Sariali, E., Hell-Remy, C., Salachas, F., Pradat, P.F., Lacomblez, L., Nicole, S., Fontaine, B., Fardeau, M., Loeffler, J.P., Meininger, V., Fournier, E., Koenig, J., Hantaï, D. Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients. Ann. Clin. Transl. Neurol., 2: 362–372, 2015; doi: 10.1002/ACN3.179
Carrasco, D.I., Bahr, B.A., Seburn, K.L., Pinter, M.J. Abnormal response of distal Schwann cells to denervation in a mouse model of motor neuron disease. Exp. Neurol., 278: 116–126, 2016; doi: 10.1016/J.EXPNEUROL.2016.02.002
Cescon, M., Gambarotta, G., Calabrò, S., Cicconetti, C., Anselmi, F., Kankowski, S., Lang, L., Basic, M., Bleich, A., Bolsega, S., Steglich, M., Oliviero, S., Raimondo, S., Bizzotto, D., Haastert-Talini, K., Ronchi, G. Gut microbiota depletion delays somatic peripheral nerve development and impairs neuromuscular junction maturation. Gut Microbes, 16: 2024; doi: 10.1080/19490976.2024.2363015
Cifuentes-Diaz, C., Nicole, S., Velasco, M. E., Borra-Cebrian, C., Panozzo, C., Frugier, T., Millet, G., Roblot, N., Joshi, V., & Melki, J. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet., 11: 2002; doi: 10.1093/HMG/11.12.1439
Cipriani, S., Phan, V., Médard, J.J., Horvath, R., Lochmüller, H., Chrast, R., Roos, A., Spendiff, S. Neuromuscular junction changes in a mouse model of Charcot-Marie-Tooth Disease type 4C. International Journal of Molecular Sciences 2018, Vol. 19, Page 4072, 19: 4072, 2018; doi: 10.3390/IJMS19124072
Desaki, J., Uehara, Y. The overall morphology of neuromuscular junctions as revealed by scanning electron microscopy. J. Neurocytol., 10: 101–110, 1981; doi: 10.1007/BF01181747
Engel, A.G., Shen, X.M., Selcen, D., Sine, S.M. Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet Neurol., 14: 420–434, 2015; doi: 10.1016/S1474-4422(14)70201-7
Frey, D., Schneider, C., Xu, L., Borg, J., Spooren, W., Caroni, P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. Journal of Neuroscience, 20: 2534–2542, 2000; doi: 10.1523/JNEUROSCI.20-07-02534.2000
Genin, E.C., Hounoum, B.M., Bannwarth, S., Fragaki, K., Lacas-Gervais, S., Mauri-Crouzet, A., Lespinasse, F., Neveu, J., Ropert, B., Augé, G., Cochaud, C., Lefebvre-Omar, C., Bigou, S., Chiot, A., Mochel, F., Boillée, S., Lobsiger, C.S., Bohl, D., Ricc, J.E., Paquis-Flucklinger, V. Mitochondrial defect in muscle precedes neuromuscular junction degeneration and motor neuron death in CHCHD10S59L/+ mouse. Acta Neuropathol., 138: 123–145, 2019; doi: 10.1007/S00401-019-01988-Z
Gessler, L., Huraskin, D., Jian, Y., Eiber, N., Hu, Z., Prószyński, T.J., Hashemolhosseini, S. The YAP1/TAZ-TEAD transcriptional network regulates gene expression at neuromuscular junctions in skeletal muscle fibers. Nucleic Acids Res., 52: 600–624, 2024; doi: 10.1093/NAR/GKAD1124
Guerra San Juan, I., Nash, L.A., Smith, K.S., Leyton-Jaimes, M.F., Qian, M., Klim, J.R., Limone, F., Dorr, A.B., Couto, A., Pintacuda, G., Joseph, B.J., Whisenant, D.E., Noble, C., Melnik, V., Potter, D., Holmes, A., Burberry, A., Verhage, M., Eggan, K. Loss of mouse Stmn2 function causes motor neuropathy. Neuron, 110: 1671-1688.e6, 2022; doi: 10.1016/J.NEURON.2022.02.011
Ham, D.J., Börsch, A., Lin, S., Thürkauf, M., Weihrauch, M., Reinhard, J.R., Delezie, J., Battilana, F., Wang, X., Kaiser, M.S., Guridi, M., Sinnreich, M., Rich, M.M., Mittal, N., Tintignac, L.A., Handschin, C., Zavolan, M., Rüegg, M.A. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nature Communications 2020 11:1, 11: 1–21, 2020; doi: 10.1038/s41467-020-18140-1
Iyer, S.R., Shah, S.B., Lovering, R.M. The neuromuscular junction: roles in aging and neuromuscular disease. Int. J. Mol. Sci., 22: 8058, 2021; doi: 10.3390/ijms22158058
Jones, R.A., Reich, C.D., Dissanayake, K.N., Kristmundsdottir, F., Findlater, G.S., Ribchester, R.R., Simmen, M.W., Gillingwater, T.H. NMJ-morph reveals principal components of synaptic morphology influencing structure–function relationships at the neuromuscular junction. Open Biol., 7: 2016; doi: 10.1098/RSOB.160240
Kennel, P.F., Finiels, F., Revah, F., Mallet, J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport, 7: 1427–1431, 1996; doi: 10.1097/00001756-199605310-00021
Martineau, É., Di Polo, A., Velde, C. Vande, Robitaille, R. Dynamic neuromuscular remodeling precedes motor-unit loss in a mouse model of ALS. Elife, 7: 2018; doi: 10.7554/ELIFE.41973
Maselli, R.A., Wollman, R.L., Leung, C., Distad, B., Palombi, S., Richman, D.P., Salazar‐Grueso, E.F., Roos, R.P. Neuromuscular transmission in amyotrophic lateral sclerosis. Muscle Nerve, 16: 1193–1203, 1993; doi: 10.1002/MUS.880161109
McMacken, G.M., Spendiff, S., Whittaker, R.G., O’Connor, E., Howarth, R.M., Boczonadi, V., Horvath, R., Slater, C.R., Lochmüller, H. Salbutamol modifies the neuromuscular junction in a mouse model of ColQ myasthenic syndrome. Hum. Mol. Genet., 28: 2339–2351, 2019; doi: 10.1093/HMG/DDZ059
Mehrotra, P., Jablonski, J., Toftegaard, J., Zhang, Y., Shahini, S., Wang, J., Hung, C.W., Ellis, R., Kayal, G., Rajabian, N., Liu, S., Roballo, K.C.S., Udin, S.B., Andreadis, S.T., Personius, K.E. Skeletal muscle reprogramming enhances reinnervation after peripheral nerve injury. Nat. Commun., 15: 9218, 2024; doi: 10.1038/s41467-024-53276-4
Mejia Maza, A., Jarvis, S., Lee, W.C., Cunningham, T.J., Schiavo, G., Secrier, M., Fratta, P., Sleigh, J.N., Fisher, E.M.C., Sudre, C.H. NMJ-Analyser identifies subtle early changes in mouse models of neuromuscular disease. Scientific Reports 2021 11:1, 11: 1–17, 2021; doi: 10.1038/s41598-021-91094-6
Minty, G., Hoppen, A., Boehm, I., Alhindi, A., Gibb, L., Potter, E., Wagner, B.C., Miller, J., Skipworth, R.J.E., Gillingwater, T.H., Jones, R.A. aNMJ-morph: a simple macro for rapid analysis of neuromuscular junction morphology. R. Soc. Open Sci., 7: 2020; doi: 10.1098/RSOS.200128
Murray, L.M., Talbot, K., Gillingwater, T.H. Review: Neuromuscular synaptic vulnerability in motor neurone disease: amyotrophic lateral sclerosis and spinal muscular atrophy. Neuropathol. Appl. Neurobiol., 36: 133–156, 2010; doi: 10.1111/J.1365-2990.2010.01061.X
Picchiarelli, G., Demestre, M., Zuko, A., Been, M., Higelin, J., Dieterlé, S., Goy, M.A., Mallik, M., Sellier, C., Scekic-Zahirovic, J., Zhang, L., Rosenbohm, A., Sijlmans, C., Aly, A., Mersmann, S., Sanjuan-Ruiz, I., Hübers, A., Messaddeq, N., Wagner, M., van Bakel, N., Boutillier, A.L., Ludolph, A., Lagier-Tourenne, C., Boeckers, T.M., Dupuis, L., Storkebaum, E. FUS-mediated regulation of acetylcholine receptor transcription at neuromuscular junctions is compromised in amyotrophic lateral sclerosis. Nature Neuroscience 2019 22:11, 22: 1793–1805, 2019; doi: 10.1038/s41593-019-0498-9
Pigna, E., Simonazzi, E., Sanna, K., Bernadzki, K.M., Proszynski, T., Heil, C., Palacios, D., Adamo, S., Moresi, V. Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine, 40: 717–732, 2019; doi: 10.1016/J.EBIOM.2019.01.038
Pratt, S.J.P., Valencia, A.P., Le, G.K., Shah, S.B., Lovering, R.M. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front. Physiol., 6: 160131, 2015; doi: 10.3389/FPHYS.2015.00252
Sakowski, S.A., Lunn, J.S., Busta, A.S., Oh, S.S., Zamora-Berridi, G., Palmer, M., Rosenberg, A.A., Philip, S.G., Dowling, J.J., Feldman, E.L. Neuromuscular effects of G93A-SOD1 expression in zebrafish. Mol. Neurodegener., 7: 1–15, 2012; doi: 10.1186/1750-1326-7-44
Sanes, J.R., Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci., 22: 389–442, 1999; doi: 10.1146/ANNUREV.NEURO.22.1.389
Schaefer, A.M., Sanes, J.R., Lichtman, J.W. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. Journal of Comparative Neurology, 490: 209–219, 2005; doi: 10.1002/CNE.20620
Sugiura, Y., Lin, W. Neuron–glia interactions: the roles of Schwann cells in neuromuscular synapse formation and function. Biosci. Rep., 31: 295, 2011; doi: 10.1042/BSR20100107
Titulaer, M.J., Lang, B., Verschuuren, J.J.G.M. Lambert-Eaton myasthenic syndrome: From clinical characteristics to therapeutic strategies. Lancet Neurol., 10: 1098–1107, 2011; doi: 10.1016/S1474-4422(11)70245-9
Tracicaru, R.-V., Bräuer, L., Döllinger, M., Schicht, M., Tillmann, B., Hînganu, D., Hristian, L., Hînganu, M.V., Paulsen, F. Morphological evidence for a unique neuromuscular functional unit of the human vocalis muscle. Int. J. Mol. Sci., 25: 11916, 2024; doi: 10.3390/ijms252211916
Tsujihata, M., Hazama, R., Yoshimura, T., Satoh, A., Mori, M., Nagataki, S. The motor end-plate fine structure and ultrastructural localization of acetylcholine receptors in amyotrophic lateral sclerosis. Muscle Nerve, 7: 243–249, 1984; doi: 10.1002/MUS.880070310
Verma, S., Khurana, S., Vats, A., Sahu, B., Ganguly, N.K., Chakraborti, P., Gourie-Devi, M., Taneja, V. Neuromuscular junction dysfunction in amyotrophic lateral sclerosis. Molecular Neurobiology 2021 59:3, 59: 1502–1527, 2022; doi: 10.1007/S12035-021-02658-6
Vieira de Sá, R., Sudria-Lopez, E., Cañizares Luna, M., Harschnitz, O., van den Heuvel, D.M.A., Kling, S., Vonk, D., Westeneng, H.J., Karst, H., Bloemenkamp, L., Varderidou-Minasian, S., Schlegel, D.K., Mars, M., Broekhoven, M.H., van Kronenburg, N.C.H., Adolfs, Y., Vangoor, V.R., de Jongh, R., Ljubikj, T., Peeters, L., Seeler, S., Mocholi, E., Basak, O., Gordon, D., Giuliani, F., Verhoeff, T., Korsten, G., Calafat Pla, T., Venø, M.T., Kjems, J., Talbot, K., van Es, M.A., Veldink, J.H., van den Berg, L.H., Zelina, P., Pasterkamp, R.J. ATAXIN-2 intermediate-length polyglutamine expansions elicit ALS-associated metabolic and immune phenotypes. Nature Communications 2024 15:1, 15: 1–25, 2024; doi: 10.1038/s41467-024-51676-0
Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nature Reviews Immunology 2002 2:10, 2: 797–804, 2002; doi: 10.1038/nri916
Watanabe, S., Davis, M.W., Kusick, G.F., Iwasa, J., Jorgensen, E.M. SynapsEM: computer-assisted synapse morphometry. Front. Synaptic Neurosci., 12: 2020; doi: 10.3389/fnsyn.2020.584549
York, A.L., Zheng, J.Q. Super-resolution microscopy reveals a nanoscale organization of acetylcholine receptors for trans-synaptic alignment at neuromuscular synapses. eNeuro, 4: 2017; doi: 10.1523/ENEURO.0232-17.2017