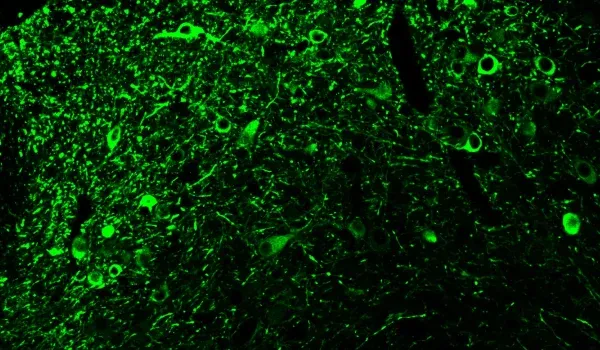
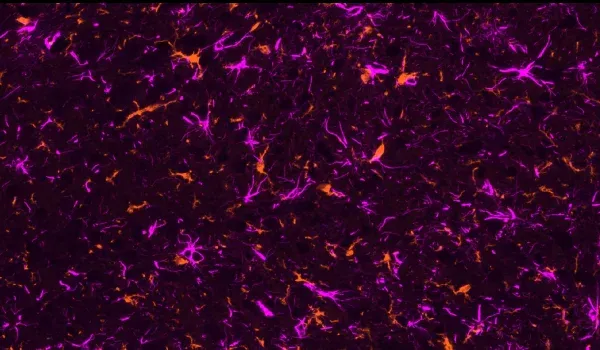
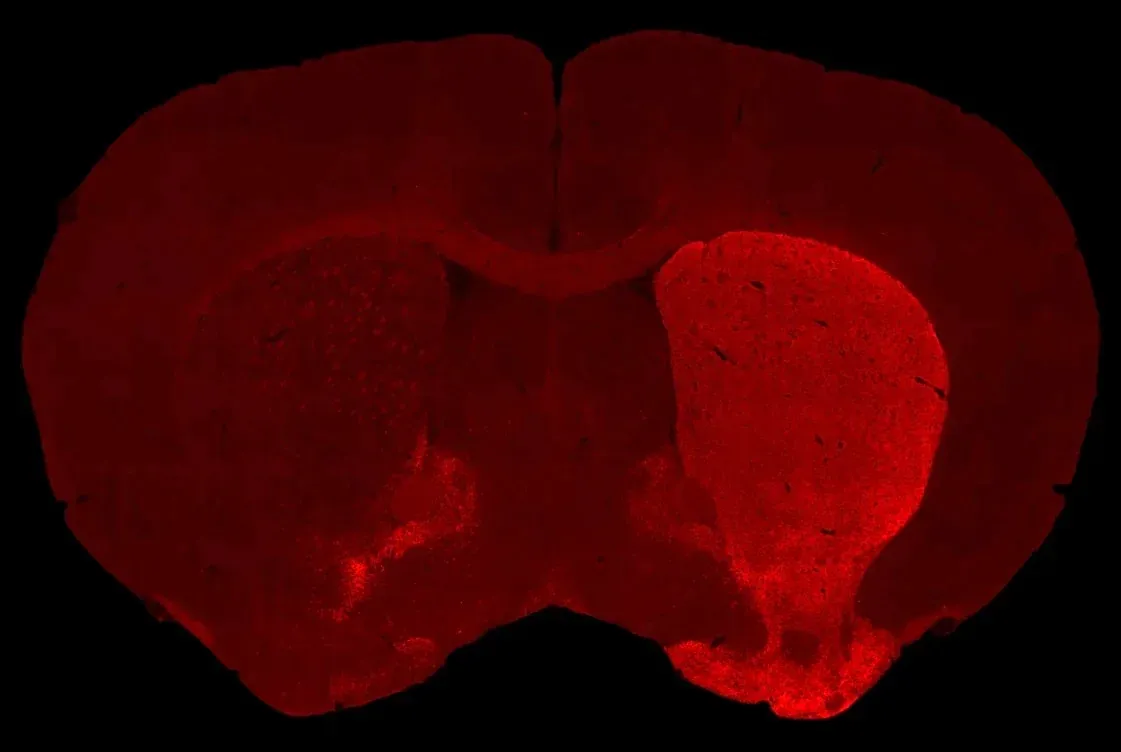
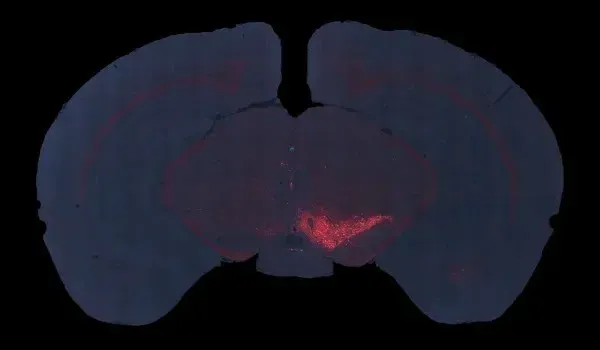
AAVタウオパチー(AAV-Tau)モデルの概要
進行性核上性麻痺および大脳基底核変性症のこのモデルでは、生後2~3ヶ月のC57BL/6マウスの黒質に、野生型ヒトタウ(MAPT)を過剰発現するAAVを片側から定位注入します。このマウスモデルは、ヒトのタウオパチーのいくつかの重要な特徴を再現しており、以下が含まれます。
- 黒質緻密部のドーパミン作動性神経細胞の減少
- 同側の線条体のドーパミン作動性神経の脱神経
- 細胞体および神経突起におけるリン酸化タウの凝集体
- 活性化されたミクログリア
- 反応性アストロサイト
- 運動機能障害
- 生体MRIスキャンで測定した脳萎縮(黒質、中脳、尾状核)
AAV-Tauモデルの生成
モデル生成の一般的なスキームは次のとおりです。
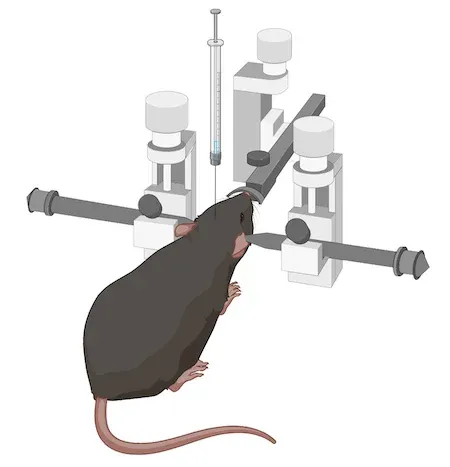
この特定のモデルでは、生後8~12週のC57BL/6マウスを使用します。次に、AAVベクターを黒質近辺に定位注入します。 高い精度と正確性を実現するために、自動マイクロインジェクター付きのデジタル定位装置を使用します。
このモデルを用いた研究は迅速に開始することができます。生体内試験は通常、約6週間続きます。そのため、特にアルツハイマー病やタウオパチーの従来のタウ遺伝子導入モデルと比較すると、比較的短期間で結果を得ることができます。
モデルの特性評価
以下のインタラクティブなプレゼンテーションでは、生体内データや多重免疫蛍光組織切片の全体像の高解像度画像など、当社のAAV-Tauマウスモデルの特性評価についてご覧いただけます。
左側のパネルを使って、この「イメージストーリー」を簡単にナビゲートすることができます。
高解像度の顕微鏡画像は、マウスの左ボタンで移動できます。 マウスまたはトラックパッド(上/下)または左上隅の + および - ボタンを使用して、拡大/縮小が可能です 。右上隅のコントロールパネル では、チャンネルとセグメンテーションの切り替え(オン/オフ)、色の変更、画像設定の調整が可能です。
最高のインタラクティブ体験 をお楽しみいただくには、フルスクリーンモード のご利用をお勧めします。
当社のAAV-Tauマウスモデルの特性評価、検証済みの測定方法、および前臨床神経科学CROサービスについて、さらに詳しく知ることができます。
当社のタウ病モデルをさらにご覧ください
関連コンテンツ
アルツハイマー病およびタウオパチーに関する最新情報と、動物モデルにおける治療薬の評価のための翻訳バイオマーカーの使用に関するベストプラクティス。
アルツハイマー病におけるアストロサイトの形態
アストロサイトの形態解析の概要と、神経変性疾患の研究および創薬・薬剤開発への応用。
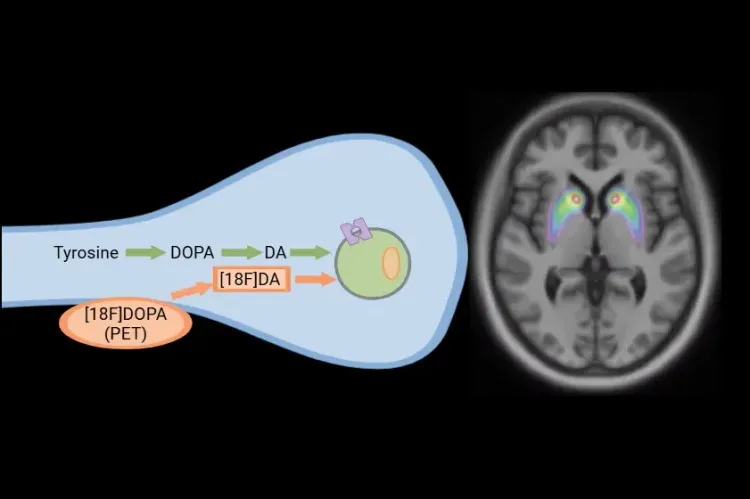
[18F]DOPA PETによるパーキンソン病臨床試験
パーキンソン病および運動障害の臨床試験における、[18F]DOPA PETによる疾患進行のモニタリングと治療介入への反応の評価方法。