Aizenstein, H. J., Nebes, R. D., Saxton, J. A., Price, J. C., Mathis, C. A., Tsopelas, N. D., Ziolko, S. K., James, J. A., Snitz, B. E., Houck, P. R., Bi, W., Cohen, A. D., Lopresti, B. J., DeKosky, S. T., Halligan, E. M., Klunk, W. E. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol., 65: 1509-1517, 2008; doi: 10.1001/archneur.65.11.1509
Arnold, S. E., Hyman, B. T., Flory, J., Damasio, A. R., Van Hoesen, G. W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex, 1: 103–116, 1991; doi: 10.1093/cercor/1.1.103
Arvanitakis, Z., Leurgans, S. E., Wang, Z., Wilson, R. S., Bennett, D. A., Schneider, J. A. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann. Neurol., 69: 320–327, 2011; doi: 10.1002/ana.22112
Braak, H., Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol., 82: 239–259, 1991; doi: 10.1007/bf00308809
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H., Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol., 112: 389, 2006; doi: 10.1007/s00401-006-0127-z
Boon, B. D. C., Bulk, M., Jonker, A. J., Morrema, T. H. J., van den Berg, E., Popovic, M., Walter, J., Kumar, S., van der Lee, S. J., Holstege, H., Zhu, X., Van Nostrand, W. E., Natté, R., van der Weerd, L., Bouwman, F. H., van de Berg, W. D. J., Rozemuller, A. J. M., Hoozemans, J. J. M. The coarse-grained plaque: a divergent Aβ plaque-type in early-onset Alzheimer’s disease. Acta Neuropathol., 140: 811–830, 2020; doi: 10.1007/s00401-020-02198-8
Busch, C., Bohl, J., Ohm, T. G. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol. Aging, 18: 401–406, 1997; doi: 10.1016/s0197-4580(97)00035-3
Byrne, U. T. E., Ross, J. M., Faull, R. L. M., Dragunow, M. High-throughput quantification of Alzheimer’s disease pathological markers in the post-mortem human brain. J. Neurosci. Methods, 176: 298–309, 2009; doi: 10.1016/j.jneumeth.2008.09.008
Cohen, M. L., Kim, C., Haldiman, T., ElHag, M., Mehndiratta, P., Pichet, T., Lissemore, F., Shea, M., Cohen, Y., Chen, W., Blevins, J., Appleby, B. S., Surewicz, K., Surewicz, W. K., Sajatovic, M., Tatsuoka, C., Zhang, S., Mayo, P., … Safar, J. G. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-β. Brain,138: 1009–1022, 2015; doi: 10.1093/brain/awv006
Crook, R., Verkkoniemi, A., Perez-Tur, J., Mehta, N., Baker, M., Houlden, H., Farrer, M., Hutton, M., Lincoln, S., Hardy, J., Gwinn, K., Somer, M., Paetau, A., Kalimo, H., Ylikoski, R., Pöyhönen, M., Kucera, S., Haltia, M. A variant of Alzheimer’s disease with spastic paraparesis and unusual plaques due to deletion of exon 9 of presenilin 1. Nat. Med., 4: 452–455, 1998; doi: 10.1038/nm0498-452
Dickson, T. C., Vickers, J. C. The morphological phenotype of β-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience, 105: 99–107, 2001; doi: 10.1016/s0306-4522(01)00169-5
Ferguson, S. A., Sarkar, S., Schmued, L. C. Longitudinal behavioral changes in the APP/PS1 transgenic Alzheimer’s Disease model. Behav. Brain Res., 242: 125–134, 2013: doi: 10.1016/j.bbr.2012.12.055
Forner, S., Kawauchi, S., Balderrama-Gutierrez, G., Kramár, E. A., Matheos, D. P., Phan, J., Javonillo, D. I., Tran, K. M., Hingco, E., da Cunha, C., Rezaie, N., Alcantara, J. A., Baglietto-Vargas, D., Jansen, C., Neumann, J., Wood, M. A., MacGregor, G. R., Mortazavi, A., … Green, K. N. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data, 8: 1–16, 2021; doi: 10.1038/s41597-021-01054-y
Greenberg, S. M., Gurol, M. E., Rosand, J., Smith, E. E. Amyloid angiopathy-related vascular cognitive impairment. Stroke, 35: 2616–2619, 2004; doi: 10.1161/01.STR.0000143224.36527.44
Han, P. L., Kim, T. K., Lee, J. E., Park, S. K., Lee, K. W., Seo, J. S., Im, J. Y., Kim, S. T., Lee, J. Y., Kim, Y. H., Lee, J. K. Analysis of differential plaque depositions in the brains of Tg2576 and Tg-APPswe/PS1dE9 transgenic mouse models of Alzheimer disease. Exp. Mol. Med., 44: 492–502, 2012; doi: 10.3858/emm.2012.44.8.056
Haroutunian, V., Perl, D. P., Purohit, D. P., Marin, D., Khan, K., Lantz, M., Davis, K. L., Mohs, R. C. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch. Neurol., 55: 1185–1191, 1998; doi: 10.1001/archneur.55.9.1185
Hulette, C. M., Welsh-Bohmer, K. A., Murray, M. G., Saunders, A. M., Mash, D. C., McIntyre, L. M. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J. Neuropathol. Exp. Neurol., 57: 1168–1174, 1998; doi: 10.1097/00005072-199812000-00009
Kapasi, A., Poirier, J., Hedayat, A., Scherlek, A., Mondal, S., Wu, T., Gibbons, J., Barnes, L. L., Bennett, D. A., Leurgans, S. E., Schneider, J. A. High-throughput digital quantification of Alzheimer disease pathology and associated infrastructure in large autopsy studies. J. Neuropathol. Exp. Neurol., 82: 976, 2023; doi: 10.1093/jnen/nlad086
Katzman, R., Terry, R., DeTeresa, R., Brown, T., Davies, P., Fuld, P., Renbing, X., Peck, A. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol., 23: 138–144, 1988; doi: 10.1002/ana.410230206
Koga, S., Ikeda, A., Dickson, D. W. Deep learning‐based model for diagnosing Alzheimer’s disease and tauopathies. Neuropathol. Appl. Neurobiol., 48: e12759, 2022; doi: 10.1111/nan.12759
Liu, P., Reichl, J. H., Rao, E. R., McNellis, B. M., Huang, E. S., Hemmy, L. S., Forster, C. L., Kuskowski, M. A., Borchelt, D. R., Vassar, R., Ashe, K. H., Zahs, K. R. Quantitative comparison of dense-core amyloid plaque accumulation in amyloid-β precursor protein transgenic mice. J. Alzheimers Dis., 56: 743, 2017: doi: 10.3233/JAD-161027
Lok, K., Zhao, H., Shen, H., Wang, Z., Gao, X., Zhao, W., Yin, M. Characterization of the APP/PS1 mouse model of Alzheimer’s disease in senescence accelerated background. Neurosci. Lett., 557: 84–89, 2013; doi: 10.1016/j.neulet.2013.10.051
Ly, P. T. T., Cai, F., Song, W. Detection of neuritic plaques in Alzheimer’s disease mouse model. J. Vis. Exp., 53: e2831, 2011; doi: 10.3791/2831
Mirra, S. S., Heyman, A., McKeel, D., Sumi, S. M., Crain, B. J., Brownlee, L. M., Vogel, F. S., Hughes, J. P., van Belle, G., Berg, L., Ball, M. J., Bierer, L. M., Claasen, D., Hansen, L. R., Hart, M., Hedreen, J., Baltimore, B., Hen Derson, V., … Terry, R. D. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology, 41: 479–486, 1991; doi: 10.1212/wnl.41.4.479
Neltner, J. H., Abner, E. L., Schmitt, F. A., Denison, S. K., Anderson, S., Patel, E., Nelson, P. T. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. J. Neuropathol. Exp. Neurol., 71: 1075-1085, 2012; doi: 10.1097/NEN.0b013e3182768de4
Oh, S. J., Lee, H. J., Kang, K. J., Han, S. J., Lee, Y. J., Lee, K. C., Lim, S. M., Chi, D. Y., Kim, K. M., Park, J. A., Choi, J. Y. Early detection of Aβ deposition in the 5xFAD mouse by amyloid PET. Contrast Media Mol. Imaging, 2018: 5272014, 2018; doi: 10.1155/2018/5272014
Ohm, T. G., Busch, C., Bohl, J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol. Aging,18: 393–399, 1997; doi: 10.1016/s0197-4580(97)00034-1
Samaroo, H. D., Opsahl, A. C., Schreiber, J., O’Neill, S. M., Marconi, M., Qian, J., Carvajal-Gonzalez, S., Tate, B., Milici, A. J., Bales, K. R., Stephenson, D. T. High throughput object-based image analysis of β-amyloid plaques in human and transgenic mouse brain. J. Neurosci. Methods, 204: 179–188, 2012; doi: 10.1016/j.jneumeth.2011.10.003
Sasaguri, H., Nilsson, P., Hashimoto, S., Nagata, K., Saito, T., Strooper, B. De, Hardy, J., Vassar, R., Winblad, B., Saido, T. C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J., 36: 2473, 2017; doi: 10.15252/embj.201797397
Selkoe, D. J., Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med., 8: 595-608, 2016; doi: 10.15252/emmm.201606210
Serrano-Pozo, A., Frosch, M. P., Masliah, E., Hyman, B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med., 1: a006189, 2011; doi: 10.1101/cshperspect.a006189
Signaevsky, M., Prastawa, M., Farrell, K., Tabish, N., Baldwin, E., Han, N., Iida, M. A., Koll, J., Bryce, C., Purohit, D., Haroutunian, V., McKee, A. C., Stein, T. D., White, C. L., Walker, J., Richardson, T. E., Hanson, R., Donovan, M. J., … Crary, J. F. Artificial intelligence in neuropathology: deep learning-based assessment of tauopathy. Lab. Invest., 99: 1019–1029, 2019; doi: 10.1038/s41374-019-0202-4
Tang, Z., Chuang, K. V., DeCarli, C., Jin, L. W., Beckett, L., Keiser, M. J., Dugger, B. N. Interpretable classification of Alzheimer’s disease pathologies with a convolutional neural network pipeline. Nature Commun.,10: 2173, 2019; doi: 10.1038/s41467-019-10212-1
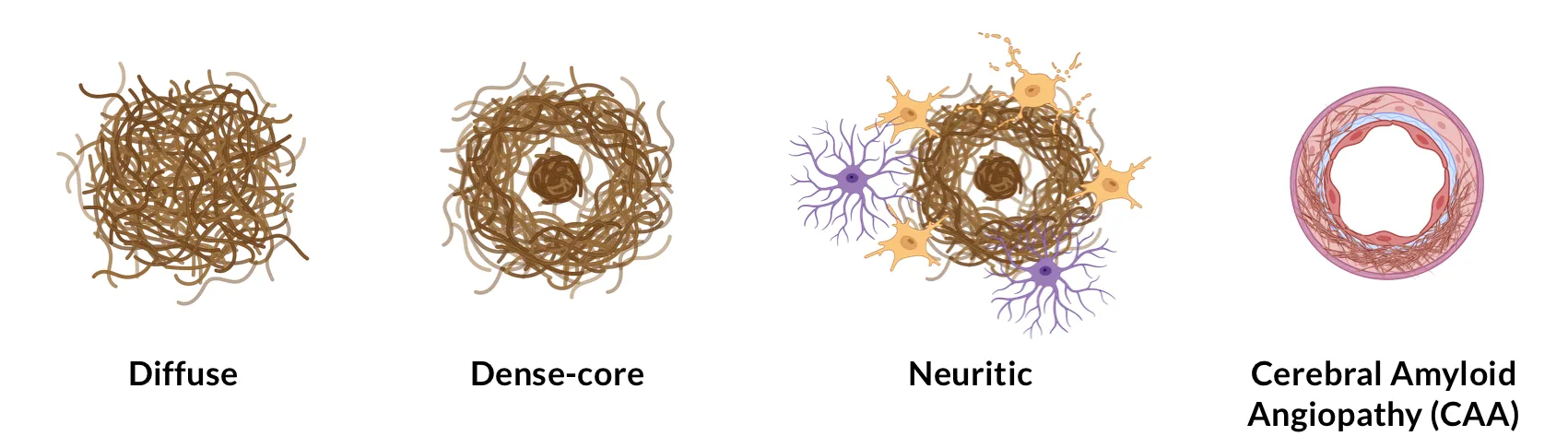
Tsering, W., Prokop, S. Neuritic plaques — gateways to understanding Alzheimer’s disease. Molecular Neurobiol., 61: 2808–2821, 2023; doi: 10.1007/s12035-023-03736-7
Vizcarra, J. C., Gearing, M., Keiser, M. J., Glass, J. D., Dugger, B. N., Gutman, D. A. Validation of machine learning models to detect amyloid pathologies across institutions. Acta Neuropathol. Commun., 8: 59, 2020; doi: 10.1186/s40478-020-00927-4
Willuweit, A., Velden, J., Godemann, R., Manook, A., Jetzek, F., Tintrup, H., Kauselmann, G., Zevnik, B., Henriksen, G., Drzezga, A., Pohlner, J., Schoor, M., Kemp, J. A., von der Kammer, H. Early-onset and robust amyloid pathology in a new homozygous mouse model of Alzheimer’s disease. PLoS One, 4: e7931, 2009; doi: 10.1371/journal.pone.0007931
Wong, D. R., Tang, Z., Mew, N. C., Das, S., Athey, J., McAleese, K. E., Kofler, J. K., Flanagan, M. E., Borys, E., White, C. L., Butte, A. J., Dugger, B. N., Keiser, M. J. Deep learning from multiple experts improves identification of amyloid neuropathologies. Acta. Neuropathol. Commun., 10: 66, 2022; doi: 10.1186/s40478-022-01365-0