Centralized Neuroradiology & Nuclear Medicine Reads
Centralized reads of MRI, PET, and SPECT images are a key element towards standardization in clinical trials. At Biospective, we specialize in operationalizing central neuroimaging reads for small (e.g. single center) to large (e.g. global, multicenter) clinical trials.
Biospective works closely with the key stakeholders (e.g. Sponsor, Clinical CRO, sites) on various aspect of the central reading process, including:
- Image acquisition protocols
- Reader selection from our network of board-certified experts
- Image Review Charter preparation (including read criteria)
- Image transfer
- Image quality control (QC) review and reconciliation
- Well-defined turnaround time for read reports
- KPIs for central review process
We use seamlessly integrated, regulatory-compliant software platforms to enable a smooth, end-to-end process. All data is meticulously tracked, and can be easily visualized for a high degree of transparency and real-time insights into our processes.
MRI Neuroradiology Reads
MRI is standard imaging modality for assessment of subject eligibility for study participation, as well as for monitoring the safety of therapeutic agents during the trial. Biospective is exclusively focused on neuroimaging in clinical trials, and our team of neuroradiologists, MRI physicists, and neuroscientists work closely with our Sponsor and their partners to define inclusion and exclusion criteria, safety measures, etc. as part of our central radiology reads services.
For specialized areas, such as Alzheimer's disease, our team has extensive expertise with centralized image reads Amyloid-Related Imaging Abnormalities (ARIA). ARIA-E and ARIA-H findings can be determined from FLAIR and T2*-weighted MRI scans, respectively. Similarly, our readers can identify White Matter Hyperintensities (WMH) and microhemorrhages as part of eligibility and safety assessments for a broad range of diseases.
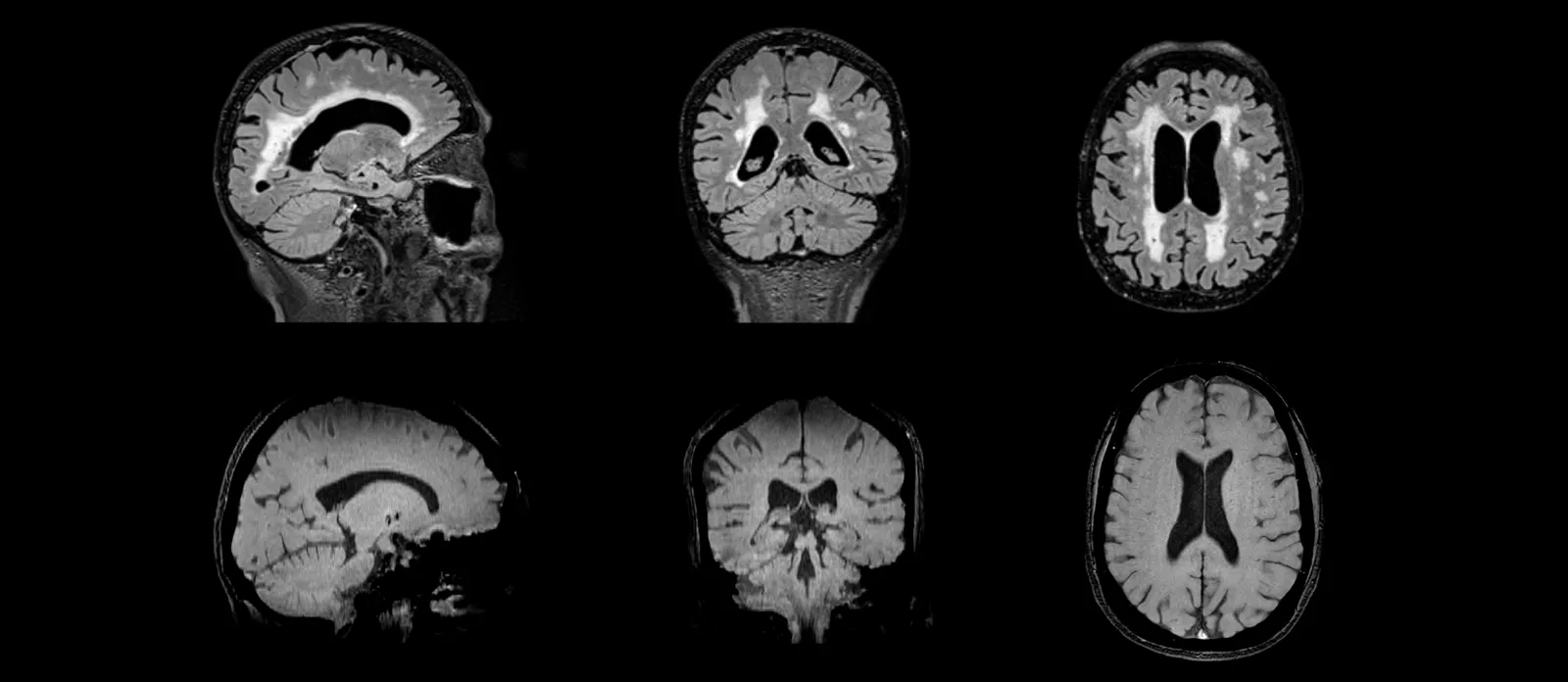
FLAIR MR images (top) for evaluation of ARIA-E and white matter hyperintensities; T2* GRE MR images (bottom) for assessment of ARIA-H and microhemorrhages.
Amyloid & Tau PET Nuclear Medicine Reads
Amyloid and Tau PET imaging using a range of radiotracers have become standard modalities in Alzheimer's disease clinical trials. We work closely with a network of internationally-recognized nuclear medicine physicians with expertise in Brain PET reading based on standardized criteria. Amyloid PET eligibility and Tau PET eligibility reads can dramatically improve subject enrichment in clinical trials involving mild cognitive impairment (MCI) and Alzheimer's disease patients at various stages of disease progression. Our team works closely with imaging sites on implementation of image acquisition protocols that are optimized for central imaging reads of these Amyloid PET and Tau PET images.
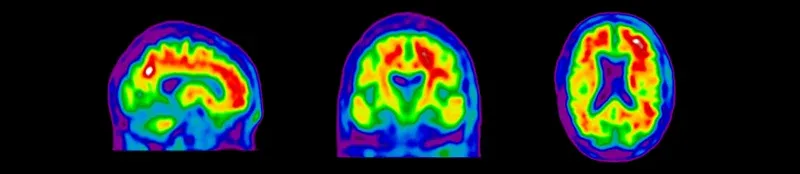 Sagittal, coronal, and axial views of amyloid PET images for eligibility screening of subjects for Alzheimer's disease clinical trials.
Sagittal, coronal, and axial views of amyloid PET images for eligibility screening of subjects for Alzheimer's disease clinical trials.Dopaminergic Imaging Nuclear Medicine Reads
Dopaminergic imaging using various PET and SPECT imaging radiotracers is now standard in Parkinson's disease clinical trials, but can be challenging to properly implement to acquire high-quality scans. At Biospective, our multi-disciplinary team of nuclear medicine clinicians, medical physicists, and image analysis scientists have established robust protocols for imaging of dopaminergic terminals. Properly reconstructed images are provided to our central readers to determine eligibility for study participation.
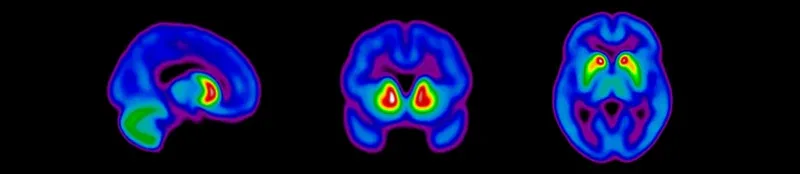 Sagittal, coronal, and axial views of dopaminergic imaging scans for eligibility screening of subjects for clinical trials of Parkinson's disease.
Sagittal, coronal, and axial views of dopaminergic imaging scans for eligibility screening of subjects for clinical trials of Parkinson's disease. Learn more about our central Eligibility & Safety Reads services for clinical trials.