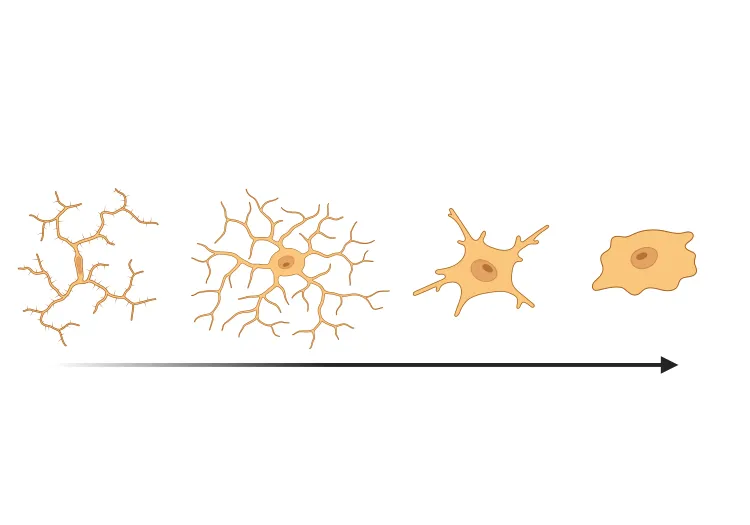
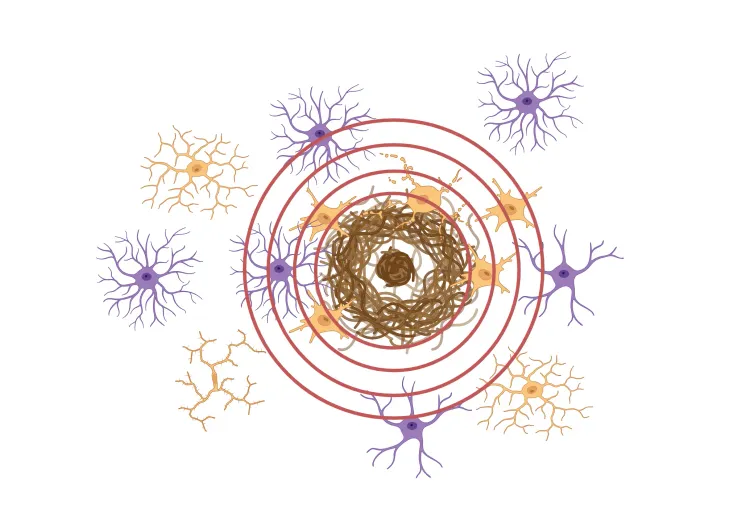
Rodent Models
Learn more about our thoroughly characterized and validated models of CNS diseases
We have a range of transgenic and inducible models readily available for your studies. Our team of animal model scientists will work with you to find the right model.
Our Services
We provide a range of specialized research services tailored to models of CNS diseases
End-to-end services from model generation to in-life testing to quantitative tissue & fluid biomarkers.
- Animal Services
- Behavioral Testing
- Electrophysiology
- Fluid & Cellular Biomarkers
- Histology & Tissue Analysis
- In Vivo Imaging
Animal Services
Our animal services team are experts in dosing via multiple routes of administration, stereotaxic surgical procedures, and fluid & tissue collection.

Behavioral Testing
We use specific, validated tests that are sensitive to response to therapy in our CNS rodent models.
Electrophysiology
We use well-established, in vivo electrophysiological measures to evaluate motor and sensory function.

Fluid & Cellular Biomarkers
We can measure clinically translational biomarkers, such as neurofilament light, in blood and cerebrospinal fluid (CSF). We can also perform cellular immunophenotyping on a range of tissues, including brain, spinal cord, lymph nodes, and spleen.
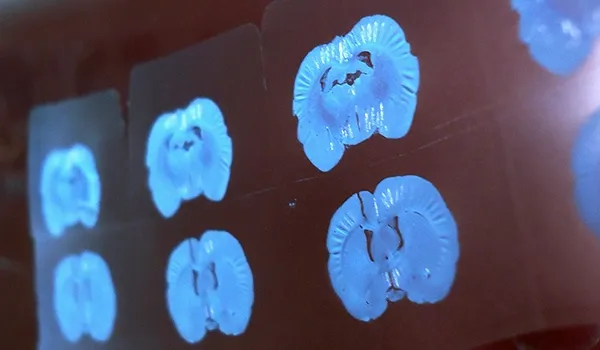
Histology & Tissue Analysis
We have a state-of-the-art histopathology facility specializing in tissues collected from rodent models of neurological diseases.
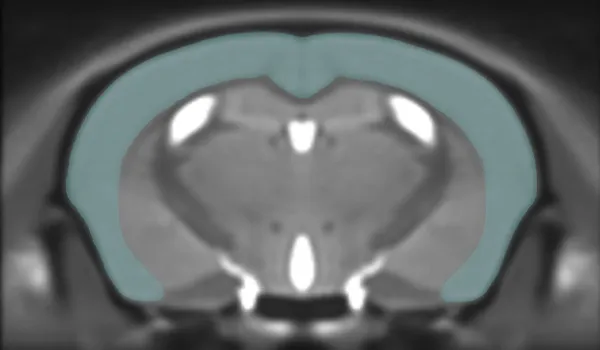
In Vivo Imaging
We use preclinical MRI, PET, and CT scanners, coupled with our advanced image processing & analysis techniques, to non-invasively measure changes in brain & muscle structure (e.g. atrophy) and function (e.g. metabolism, blood flow).
Our Latest Innovations
Narrated presentations of our scientific advancements
Microglial activation in an α-synuclein mouse model of Parkinson’s disease
Updated Oct 16, 202310 min presentationLeveraging computer vision and machine learning algorithms, we have quantified microglial activation in immunohistochemistry (IHC) sections from an α-synuclein preformed fibril (PFF) seeding & spreading mouse model.
β-amyloid & the inflammatory microenvironment in an APP/PS1 mouse model of Alzheimer’s disease
Updated Dec 14, 202310 min presentationBy applying advanced image analysis techniques to multiplex immunofluorescence data, we have been able to explore spatiotemporal changes in the inflammatory microenvironment of β-amyloid plaques in an APP/PS1 transgenic model.
Brain atrophy analysis in rodent models of neurodegeneration
Updated Dec 18, 202310 min presentationBrain atrophy is a hallmark of many neurodegenerative diseases. MRI is routinely used in clinical trials to non-invasively measure volumetric and cortical thickness changes. We have applied these translational imaging biomarkers to mouse models of ALS and Parkinson’s disease.